
The authors present an update to the Wyeth/BASF experience with the IPEC Novel Excipient Safety Evaluation Procedure.

The authors present an update to the Wyeth/BASF experience with the IPEC Novel Excipient Safety Evaluation Procedure.

Stuart Needleman, president of active-ingredient development and manufacturing at Aptuit, discusses industry trends and challenges.

The author reviews key considerations for formulating powders for use in inhalers. This article is part of a special Drug Delivery issue.

Microbubbles can temporarily open many biological barriers for polar molecules, macromolecules, and particles. Scientists have brought well-known contrast agents back to the laboratory and redesigned them as drug carriers. This article is part of a special Drug Delivery issue.

A review on the current status of long-acting injectables, including commercially marketed products. This article is part of a special Drug Delivery issue.

The authors describe an alternative approach to compressing and coating minitablets for use in a sustained-release, solid oral-dosage form. This article is part of a special Drug Delivery issue.

The author outlines how to choose carriers and capsule shells according to dosage requirements and intended use. This article is part of a special Drug Delivery issue.
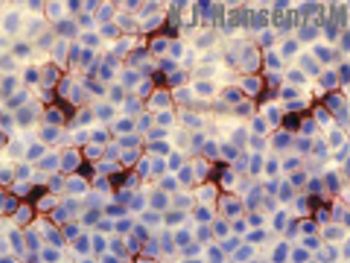
The author reviews advances in technology that may soon allow transdermal delivery of two of the fastest growing drug classes on the pharmaceutical market. This article is part of a special Drug Delivery issue.

Although there are no regulatory requirements or established pharmacopoeial techniques for the dissolution testing of inhaled drugs, such testing can potentially open up the opportunity to tailor formulation properties.

This article examines the difficulties in designing lyophilisation processes that can be faithfully scaled up to production volumes and suggests the most effective ways in which this can be achieved.

PPD and Bend Research Form Collaboration; Ricerca Makes Senior Appointment; And More.

Pfizer Forms Pact with Biocon; Baxter Makes Appointments; And More.

sanofi to Cut 25% of US Workforce; AMRI Names VP of Chemical Development; And More.

Company and People Notes: Novartis Settles with US Attorney's Office; Hospira Names VP, And More.

The reopened debate over embryonic-stem-cell research could stifle many other scientific pursuits.

A recently released industry guide outlines a science- and risk-based approach to control the risk of cross-contamination.

Private companies and universities are developing new ways to deliver protein drugs.

Public-private R&D partnerships are on the rise across Europe, but national goals and academia-industry competition could prevent their success at the European level.
Drugmakers hatch new manufacturing paradigms in the wake of the 2009 H1N1 influenza pandemic.

From disagreement to denial, being cordial about quality control can be challenging.

In light of compendial changes, representatives of the US Pharmacopeia and an industry consortium provide perspectives on cap and ferrule labels.
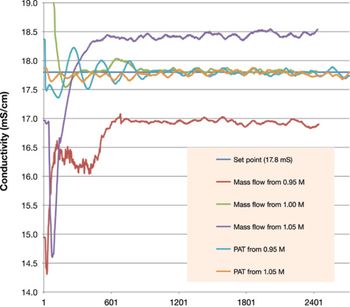
The authors describe the operational requirements and design of a process-ready PAT-based IBD system.

The author describes the development of small-angle X-ray scattering and analyzes its advantages in the characterization of drug-delivery systems and large molecules. This article is part of a special Analytical Technology issue.

Novartis Sells US Enablex Rights; Ricerca Names Chemistry Director; And More.

DSM and PolyTherics in Development Deal; Kite Pharma Appoints President and CEO, And More.