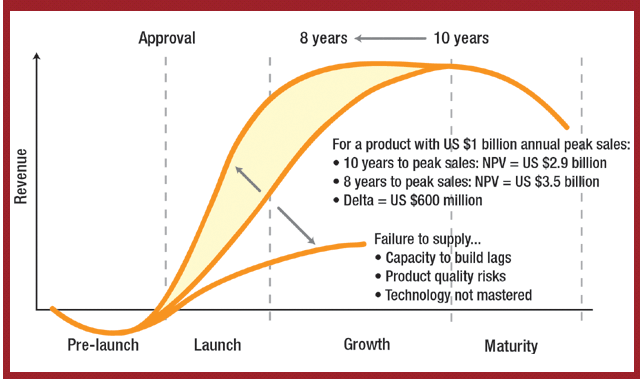
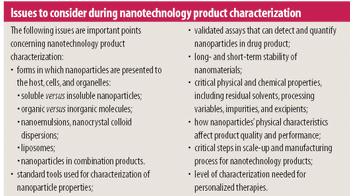
The US Food and Drug Administration (Rockville, MD) has sent warning letters to RoTech Healthcare, Inc. (Orlando, FL), CCS Medical (Clearwater, FL), and Reliant Pharmacy Services (Clearwater, FL), demanding that they stop manufacturing and distributing compounded, unapproved respiratory drugs.