
von Eschenbach Drops NCI Hat…for Now

von Eschenbach Drops NCI Hat…for Now

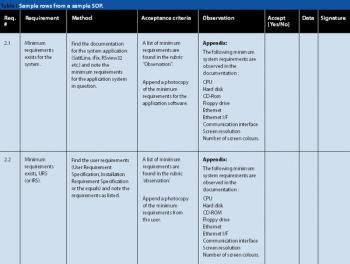
Formulation residue, observer viewing distance, light intensity, viewing angle, observer viewing position, and observer-to-observer variability affect the ability to confirm the cleanliness of manufacturing equipment.
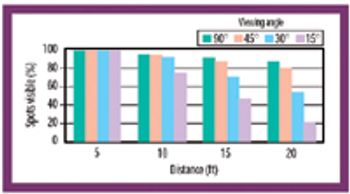
Formulation residue, observer viewing distance, light intensity, viewing angle, observer viewing position, and observer-to-observer variability affect the ability to confirm the cleanliness of manufacturing equipment.
The US Food and Drug Administration (Rockville, MD, www.fda.gov) released a new draft guidance that may speed generic approvals. The guidance, ANDAs: Impurities in Drug Products, describes the degradation-product information that generic drug manufacturers should include in their abbreviated new drug applications (ANDAs). This clarification, FDA officials say, will help companies submit the correct information, thus increasing the likelihood that their generic drugs will be approved, and approved more quickly.
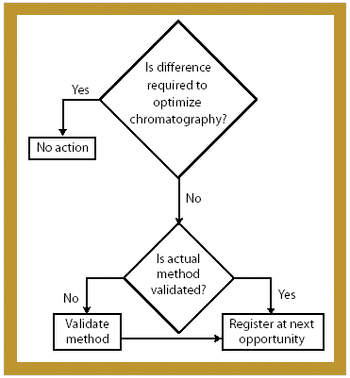
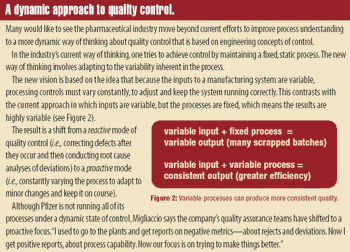
Manufacturers can take steps to establish a regulatory compliance assessment program in their pharmaceutical manufacturing facilities.

Ensuring access to quality medicines while ramping up production requires attention to supply chains, bioequivalence testing, and patent and regulatory issues.
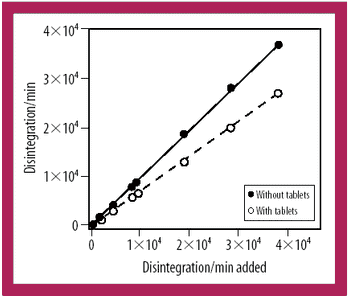
A radiotracer technique is a simple, fast, and sensitive technique for analyzing the integrity of clinical supply packages to water.

It was, our GMP Agent-in-Place recalls, a typical, small, clinical-type facility... managed in the typical, informal way.
In the pharmaceutical world it is generally accepted that a valve can be exchanged for another when it becomes defective, provided that the new one is exactly the same as the old one.

Chemical purity is the most important quality characteristic of a pharmaceutical substance. This article describes the latest scientific and technological advances to meet recent pharmacopoeial and regulatory requirements regarding the control of organic impurities in synthetically produced active substances. Future developments and suggestions for those working in quality control and raw material selection are discussed.

FDA Proposes PET CGMPs

FDA Places All Andrx ANDAs on Hold

Warning Letter: Similasan

The US National Institute of Allergy and Infectious Diseases (NIAID, Bethesda, MD, www3.niaid.nih.gov) announced it will order two million doses of an avian influenza vaccine from Sanofi-Pasteur (Swiftwater, PA and Lyon, France, www. sanofipasteur.com). In April, the NIAID began a Phase I trial to evaluate the vaccine’s safety and ability to generate immunity against the H5N1 strain of avian flu, an illness that leads to severe disease and possible death in birds and humans.

Technology's new monthly "Agent-in-Place" column distills true-life cautionary tales from the secret files of Control, a senior compliance officer.

Commissioner Crawford’s top priority is to restore public confidence in FDA oversight of drug safety and quality.

Recalls: Trypan Blue 0.06% Ophthalmic Solution and Alcohol-Free Mouthwash

New Guidance May Speed Generic Approvals

The European Commission has released its second report to the Council and European Parliament addressing the developments and implications in patent law concerning biotechnology and genetic engineering. The study, which centres on the patentability of inventions relating to stem cells, concluded that those capable of developing into human beings, totipotent stem cells, are to be excluded from patentability on the Directives' grounds of human dignity.

Warning Letter: Cape Drugs

Warning Letter: Merck & Co.

Chiron Corporation (Emeryville, CA, www.chiron.com) announced that quality problems at its Marlburg, Germany, manufacturing plant will prevent the company from supplying its "Begrivac" influenza virus vaccine to non-US markets for the 2005–2006 flu season.

Although new drug development usually focuses on clinical and preclinical research, moving innovative products from clinical testing to market mainly involves overcoming manufacturing capabilities and production challenges. Ensuring access to consistently high-quality critical vaccines and therapies needed to counter bioterrorism attacks is a topic frequently debated. Product shortages are leading to policies that expand US drug and vaccine manufacturing and ensure that US regulatory and healthcare policies avoid erecting roadblocks to high-quality drug production.

Our GMP Agent-in-Place at a top-10 pharmaceutical manufacturing firm reports on a spill during the manufacture of a time-release capsule filled with coated beads.
In 1987, when the US Food and Drug Administration issued its Guideline on General Principles of Process Validation, a young FDA reviewer asked her supervisors.