
French pharma giant Les Laboratoires Servier SA has been accused of providing "misleading" and "incorrect" information during the EC's antitrust investigation.

French pharma giant Les Laboratoires Servier SA has been accused of providing "misleading" and "incorrect" information during the EC's antitrust investigation.

In 2009, the European Commission (EC) Customs Union seized 11,462,533 medicines and medical products for suspected violations of intellectual property (IP), according to the Commission's annual report on EU Customs Enforcement of Intellectual Property Rights, which was published last week.

European Medicines Agency Issues Drug Review Update; And More

The US Food and Drug Administration has joined the Tox21 collaboration, which aims to develop ways to more effectively predict how chemicals will affect the body and environment.

The pharmaceutical industry?s increasing interest in inhaled drugs has prompted several researchers to propose standard dissolution-testing methods for these products.

The Council of Europe (CoE) is hoping its 'Medicrime' treaty can help curb the lucrative global trade in fake medicines.

States can reduce their Medicaid programs' healthcare expenditures by changing laws to enable generic drugs to be substituted for branded medications more easily and quickly, according to a new study conducted by CVS Caremark, a large pharmacy healthcare provider.

The Laboratory Services Division of the Philippine Food and Drug Administration (FDA) has attained internationally recognized accreditation for its testing and calibration laboratories, according to a July 12, US Pharmacopeia (USP) announcement.

The Council of Europe (CoE) is hoping its Medicrime treaty can help curb the lucrative global trade in fake medicines.

The Pharmaceutical Research and Manufacturers of America (PhRMA) announced earlier this week that John J. Castellani will replace Billy Tauzin as President and Chief Executive Officer.

Crunch time is rapidly approaching in Roquette?s long-running patent dispute with SPI Pharma, with the case scheduled to go to trial on 4th October 2010.

Changes to Part 2 of the EU GMP guide will come into force by 31 July 2010 in order to bring it in line with the ICH Q9 guideline on Quality Risk Management. As a result, it will no longer be identical to the ICH Q7 guideline on APIs - a harmonised guideline for the US, Europe and Japan.

Last week, Senator Charles Grassley (R-IA) sent letters to 16 drugmakers, including Pfizer (New York), AstraZeneca (London), and Eli Lilly (Indianapolis), asking them about their current policies regarding whistleblowers?employees who file complaints under the False Claims Act (FCA).

A history of the selection of the widely used significance level leaves much to be desired.

To continue innovating, the biopharmaceutical sector needs support from all levels.

As generic divisions become the most-wanted acquisitions of Big Pharma, India's domestic industry may be thinning out.
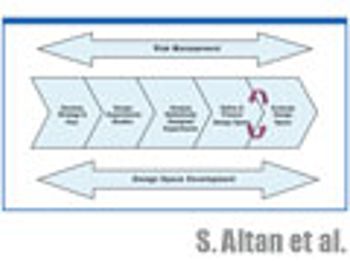
The authors discuss the statistical tools used in experimental planning and strategy and how to evaluate the resulting design space and its graphical representation.

Reports of overlooked controls, dropped pallets, and misplaced documents leave a chill in the air.

After a spate of industrial disasters, the public seeks greater oversight of corporations-so does FDA.

More information may be released to improve public understanding of regulatory policies.

Pfizer Suspends Tanezumab Program; Actavis Appoints CEO; And More.

The US Food and Drug Administration issued on June 25 a draft guidance for industry, "CMC Postapproval Manufacturing Changes Reportable in Annual Reports," to provide recommendations to drug applicants about the types of changes that may be included in annual reports.

The Office of the Inspector General for the Department of Health and Human Services (HHS) issued a report, Challenges to FDA's Ability to Monitor and Inspect Foreign Clinical Trials, which evaluates the incidence of foreign clinical trials, the ability of the US Food and Drug Administration to oversee these trials, and recommendations to FDA on how to improve its oversight of foreign clinical trials.

Europe needs a more standardised and consistent approach for supplying excipient information to the regulators.

The effects of counterfeiting are hard to measure, both in human impact and financial loss.