
In order to combat the threat of counterfeits in Europe, the EU's current anti-counterfeiting directive is being amended.

In order to combat the threat of counterfeits in Europe, the EU's current anti-counterfeiting directive is being amended.
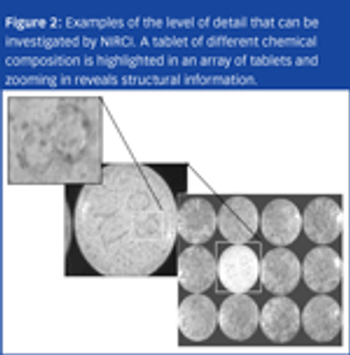
Counterfeit pharmaceuticals are complex products that can vary from their legitimate counterparts both chemically and physically.
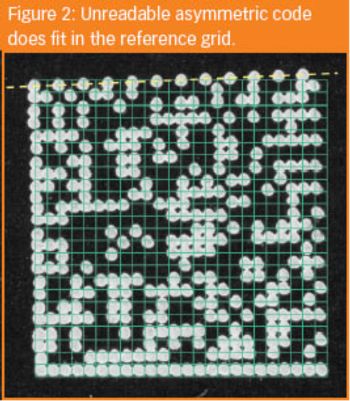
Wyatt Earp, the legendary sheriff of Tombstone used to solve troubles in a simple way: aiming, pulling the trigger and bang! ... problem eliminated! Some manufacturers check the readability of their barcodes in the same way.

Teva Pharmaceuticals in the US has admitted to including false statements in the physician prescribing information for its oral contraceptive product Gianvi, a generic version of Bayer Healthcare?s YAZ oral contraceptive.

A study conducted by the European Medicines Agency (EMA) has shown that statistical methods using EMA's EudraVigilance database of adverse drug reaction reports can be used to detect drug safety issues "significantly earlier" compared with routine pharmacovigilance.

Regulatory Roundup: USP and Russia's Roszdravnadzor Sign MOU

The US Food and Drug Administration began a partnership with the website Drugs.com to expand access to the agency's consumer-health information.

The US House of Representatives held hearings last week to gain testimony on the potential benefits and risks associated with synthetic biology and synthetic genomics.

About one month after the announcement of McNeil Consumer Healthcare's recall of children's liquid pain and allergy medications , the US Food and Drug Administration testified before the US House Committee on Oversight and Government Reform about the issue.

USP membership meeting prepared the standards-setting body to meet modern challenges.

Europe moves to place excipient GMP and GDP standards on the same level as active pharmaceutical ingredients.

Too much or too little control can actually lead to the same result.
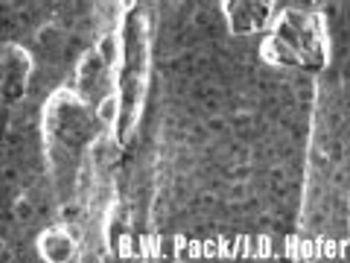
The authors recommend a strategy for classifying similar nonstainless-steel surfaces into three groups based upon the analytical recovery that was observed in this study.


An effective quality management system will identify the root cause of non-conformances and put measures in place to ensure that they do not recur, but is the pharmaceutical industry choosing the right methods to make sure this happens?

Shared audits of suppliers offer several advantages, but networking to conduct such an audit can be challenging.

Genzyme (Cambridge, MA) signed a consent decree of permanent injunction that requires the company to correct manufacturing-quality violations at its Allston, Massachusetts, manufacturing facility.

More acquisitions are expected as the patent cliff pushes the pharma industry into a critical phase, with many executives believing that the industry will be unable to innovate sufficiently from within to replace blockbuster drugs.

Lonza Acquires MODA; Sigma-Aldrich Exec to Retire; And More.

The International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) published its "Ten Principles on Counterfeit Medicines" last week to draw public attention to the issue.

The US Food and Drug Administration launched a program last week designed to "educate healthcare providers about their role in ensuring that prescription drug advertising and promotion is truthful."

Online process monitoring could help companies achieve the dual goals of ensuring high end-product quality and satisfying regulatory demands.

Effective May 1, 2010, the US Pharmacopeia has revised its Pending Monographs Guideline to clarify that excipients are eligible for pending-monograph status and can ultimately be included in an official National Formulary (NF) monograph.

Sundlof Resigns as FDA's Head of Food Safety; and More

In an effort to standardize quality agreements for active pharmaceutical ingredients in the drug industry, the Bulk Pharmaceuticals Task Force, an affiliate of the Society of Chemical Manufacturers and Affiliates, has developed a template to help manufacturers and customers comply with US regulatory requirements in a simplified manner.