
Johnson & Johnson's run of quality issues, recalls and other bad news continues with yet another product recall.

Johnson & Johnson's run of quality issues, recalls and other bad news continues with yet another product recall.

Merck Teams with Parexel; Roche CFO to Retire; and More

FDA Sets Limits for Acetaminophen in Prescription Combination Products

The EU Commission is on the lookout for "potentially problematic patent settlements" and has asked several pharma companies to submit copies of their patent settlement agreements made between originator and generic companies.

FDA issued a draft guidance for industry on Jan. 18, 2011, about the size of beads within drug products labeled for sprinkle.

GlaxoSmithKline is expecting to pay $3.4 billion to settle legal charges relating to its diabetes drug Avandia, as well as sales and promotional practices in the US for other products.

With counterfeit medicines increasingly appearing in legitimate supply chains and even in the clinical stages of product development, a recent white paper has outlined practical steps that may help companies address the issue of counterfeits and diverted products.

Urgent changes are required in the UK's regulation and governance of health research because medical advances are currently being stifled by unnecessary delays, bureaucracy and complexity.

Last week, the US Pharmacopeia published proposed standards for the content, language, format, and appearance of prescription drug labels in an effort to enhance patient understanding and safety.

As part of its transparency initiative, which began in June 2009, FDA launched a website during the first week of January 2011 that addresses basic questions for industry about regulated products.

Direct-to-consumer (DTC) advertising is firmly entrenched in the US, but has historically been considered as an inappropriate means of communication in Europe for prescription-only medicines.

Iain Moore explains what progress has been made so far regarding EU plans to regulate excipients.

The threat of counterfeit medicines in the next year will be more severe than ever, according to a survey of 1000 companies conducted in October 2010 by Pharma IQ.

Excipients should be regulated to manage risk. Although, excipients are an integral part of a finished pharmaceutical product, the issue of regulation is complex.

GlaxoSmithKline (GSK) issued a statement on Jan. 2, 2011, to respond to a 60 Minutes segment that aired on Jan. 2 that focused on quality-control problems at a former GSK manufacturing facility in Cidra, Puerto Rico.

Gilead Sciences to Acquire Arresto Biosciences; Sartorius Appoints Head of Lab Business; and More.

John Taylor to Serve as Acting Principal Deputy Commissioner after Joshua Sharfstein's Departure, and more.

US Senator Amy Klobuchar (D-MN) urged the US Food and Drug Administration and the pharmaceutical industry to address what she called an "unprecedented" shortage of prescription drugs, especially for chemotherapy.

FDA received the authority to mandate recalls of food under a new food-safety bill. Efforts are underway in the House to grant the agency this power for pharmaceuticals as well.

The European Commission and Medicines Agency seem to be moving in advance of their ICH partners to update standards.

Expert and implementation working groups harmonize more guidelines and move Q11 forward.

The authors discuss how their research will help FDA in its identification of areas of emphasis in pre- and postapproval evaluation of products and processes.

Top priorities for manufacturers include user fees, new health initiatives, and regulatory compliance.
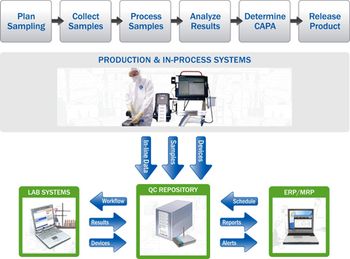
The authors discuss the role of quality-control automation in providing better data, enhanced compliance, and potentially faster release times.

Capable of great works, pharma as a whole still yields to the lesser angels of its nature.