
New Web Search Feature on FDA.gov, Search for Product Recalls More Quickly

New Web Search Feature on FDA.gov, Search for Product Recalls More Quickly

Johnson & Johnson has instituted a new structure for its Consumer Group according to a Reuters report.


The Society of Chemical Manufacturers and Affiliates criticized the Secure Chemical Facilities Act, which would require chemical facilities to use inherently safer technology as part of chemical-site security measures.

Indian manufacturers are not a near-term threat to Western CMOs, but may be long term.

Courts and Congress seek to reshape policies and programs.

Research and development may be headed for divorce.

The contract-research industry in China is growing in leaps and bounds, and Big Pharma is leading the way.

Regulators question whether particles that they can't see hurt patients.

PhRMA efforts of industry's R&D scientists.

A review of current efforts within PDA's Paradigm Change in Manufacturing Operations initiative.

In any industry, inspections can be a pain, and pharma is no exception.

Companies engaged in global mergers and acquisitions may be hearing from the Department of Justice more often to ensure that corruptive practices are not taking place.

The need for greater process understanding raises the bar for suppliers.

The hardest errors to spot are the ones that don't look like errors at all.

Bob Weaver, president of HunterLab, discusses current trends and challenges.

This technical forum is part of a special issue on Solid Dosage and Excipients.
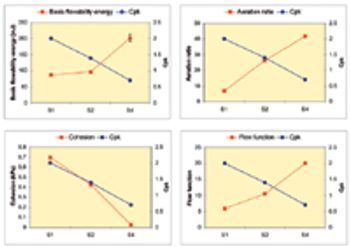
The author explains how to gain an understanding of the relationships between powder characteristics and process performance to match filling-machine geometry to the demands of specific formulations.

The author focuses on how industry can build a system for Total Excipient Control.
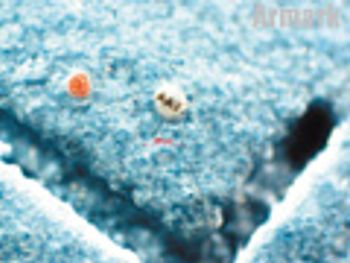
Representatives from Pfizer R&D, DEM Solutions, Colorcon, and ARmark Authentication Technologies provide insight into recent tablet-coating technologies.

Europe's approach to pharmacovigilance has room for improvement that European agencies are working on.
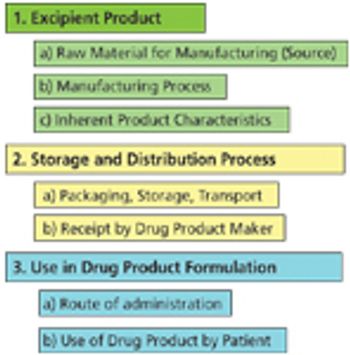
The author describes key considertions for a complete risk-assessment model and provides insight into a pending IPEC guideline in this area.

Nanotechnology often is associated with parenteral drug delivery, particularly for anticancer therapies, but it also has applications in oral drug delivery

The author reviews Warning Letters issued between 2000 and mid-2010 for aseptic processing and non-sterile processing, and determines how many observations were made for each section of the GMPs.
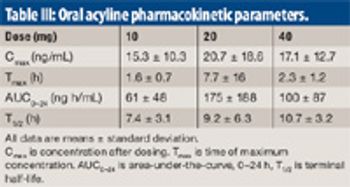
The authors examine an oral-absorption-enhancement technology based on surface-active materials to increase apical membrane fluidity in vitro.