
If not properly monitored, filters and plastic bags can keep back more than they should.

If not properly monitored, filters and plastic bags can keep back more than they should.

The United States Pharmacopeia emphasizes mechanical calibration and a performance test to esnure integrity of the dissolution procedure.
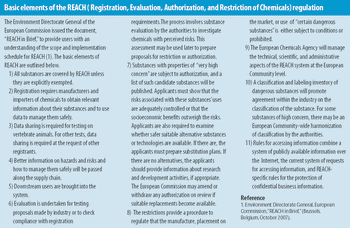
The European Union's REACH initiative has the potential to affect the flow of chemicals into the pharmaceutical suppy chain.

Show blasts off this month in Philadelphia with more suppliers, new trends, and real-world solutions.

The US Food and Drug Administration released a final guidance on protocol for testing sterile products.

The US Food and Drug Administration has issued generics manufacturer Vintage Pharmaceuticals a warning letter stemming from July through August 2007 that found observations pertaining to microbial contamination and failures in testing and documentation procedures.

Also, Biocon will acquire 70% of AxiCorp, ARIAD Pharmaceuticals promoted Richard W. Pascoe to the new position of COO, more...

Baxter Healthcare Corporation is providing an update to its January 2008 heparin sodium injection 1000 units/mL 10 and 30mL multi-dose vial voluntary recall of nine lots, which the company initiated as a precautionary measure due to an increase in reports of adverse reactions that may be associated with the drug.

The US Food and Drug Administration is requesting a 5.7% increase in its budget between the current fiscal year, FY 2008, and FY 2009, for a total $2.4 billion budget.

Amira and GSK Form Agreement, Xenome Appoints Ian Nisbet CEO, More...

The US Food and Drug Administration posted on its website a Jan. 24 Warning Letter to Novartis Vaccines and Diagnostics.
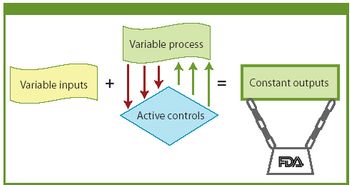
FDA is modernizing and streamlining current good manufacturing practices. The author examines FDA's evolving approach to quality systems and how a manufacturer can implement a quality system framework.

The rise in overseas manufacturing undermines FDA oversight of drug quality.

PharmTech's polls feature user feedback on issues facing the pharmaceutical industry.

USP 467 Residual Solvents will take effect on July 1, 2008. But does the industry understand these specifications-and is it prepared?

Can an overload of patent applications lead to the US' demise as a scientific leader?

If at first the product fails, then inspect, inspect again

A wave of pharmaceutical expansions is expected in Europe this year, surprisingly by Indian companies.

Connecticut Attorney General Richard Blumenthal is investigating Schering-Plough (Kenilworth, NJ) and Merck & Co. (Whitehouse Station, NJ), according to a Reuters report.

Based on its investigation into the safety of over-the-counter cough and cold drug products for children, the US Food and Drug Administration is recommending against such products for all children under two years of age.

Congressmen John Dingell and Bart Stupak have requested that the Government Accountability Office perform an updated assessment report in response to the US Food and Drug Administration?s potential development of a new class of behind-the-counter drugs.

The US Food and Drug Administration published a draft annex to its ICH Q8 Pharmaceutical Development guidance that clarifies that document?s key concepts.

Noven Pharmaceuticals received a warning letter from the US Food and Drug Administration stemming from an on-site inspection of the company?s manufacturing facility in Miami, Florida, concluded July 2007.

The US Food and Drug Administration?s website will no longer allow the public to electronically submit comments to its dockets.

The US Food and Drug Administration approved 69 new drug applications last year, the second lowest number of approvals in the past decade, according to the Jan. 15 edition of Drug Industry Daily.