
The draft guidance ICH Q10 for pharmaceutical quality systems is part of the ongoing move to a science- and risk-based approach in manufacturing.

The draft guidance ICH Q10 for pharmaceutical quality systems is part of the ongoing move to a science- and risk-based approach in manufacturing.

Useful Contacts

Poor processing and misguided projections lead to trashed product.

In an age of "pre-existing conditions," can a new bill help patients get the treatment they need?

Larger and strategic sampling and testing plans can improve process understanding and characterization.

The recently published Orange Guide 2007 contains significant changes to the GMP requirement placed on pharmaceutical manufacturers, but there have been additional changes to good distribution practice that should not be overlooked.

The authors discuss how companies facing ever-evolving regulatory requirements can address and assess compliance risk in their operating practices.

As China emerges as a significant supplier of pharmaceutical ingredients, it must assure other countries of the safety of its excipients.

Campaigners for Alzheimer's sufferers to have access to antidementia drugs on the NHS in the early stages of the disease have claimed a small victory in this drugs battle.

Feedback is urged on USP's proposed revisions to the General Notices.

FDA's long-awaited GMPs for supplements appear as food and drug safety concerns override lingering opposition.

Did you know that only one-third of prescribed pediatric drugs have been studied or labeled for pediatric use? The Best Pharmaceuticals for Children Act (BPCA) was enacted in 2002 to improve this statistic by providing companies with six months of additional marketing exclusivity if they conduct pediatric trials. The Government Accountability Office recently issued a report that examined data from studies conducted between 2002 and 2005 for drugs specified by the US Food and Drug Administration under BPCA.

Production sometimes follows the law of supply and reprimand.

Washington, DC (June 25)-More than five million US adults import prescription drugs from other countries, two million of them without an official prescription, according to a survey conducted by the Pharmaceutical Research and Manufacturers of America (PhRMA). Concerned about the number of counterfeit drugs entering the US, PhRMA launched the survey to determine who was importing prescription drugs and why.

USP is revising key documents to make them easier to use.

Industry and regulatory experts provide advice on inspection preparation and best practices.
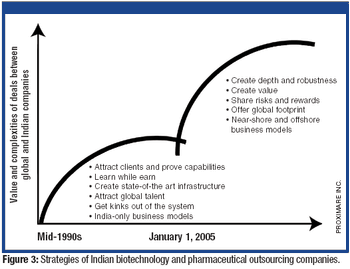
The tightening of intellectual property rights in India under GATT/TRIPS was a crucial inflection point for pharmaceutical outsourcing in India.

This article is written to assist clinical manufacturing representatives at pharmaceutical companies who are faced for the first time with outsourcing the manufacture of clinical supplies. The author describes the identification, writing, and execution of documents required to support the contract manufacture of products for clinical studies.

A new guidance issued by the US Food and Drug Administration earlier this month advises companies on how to treat polymorphic drug compounds?those that exhibit multiple structural forms?in filing abbreviated new drug applications.
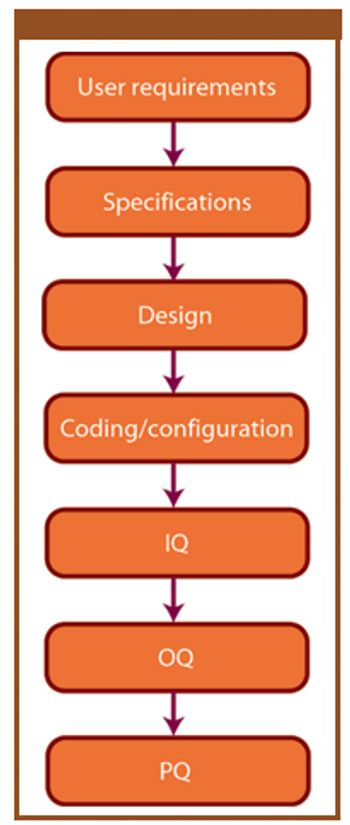
This article provides a historical review of computer validation in the pharmaceutical industry within the last three decades, evolving from the early years' initial concept and approach to today's current practices. Also included is how the regulations and industry have progressed in addressing the topic of computer validation.

This article looks at the current good manufacturing practice regulations from a statistical perspective while addressing their requirements and implications and inviting the industry to assess its past performance in meeting the regulations.

The quality of a drug product is an essential element of drug safety and efficacy. With a statutory mission to provide safe and effective medications to the public, the US Food and Drug Administration has always focused on drug quality. The authors summarize the history of FDA's role in ensuring product quality and its role in shaping risk-based approaches to this goal.

Here's a look at some of the most interesting and thematic responses you-our readers-provided for Pharmaceutical Technology's anniversary survey of industry advances and directions.

The good ol' days weren't always good.

The US Food and Drug Administration has pursued many new initiatives since 1977. The agency began accepting abbreviated applications for generic versions of drugs, collected user fees to support drug review, launched the Critical Path Initiative to encourage innovation, and worked to harmonize its regulatory standards with those of Europe and Japan. New challenges such as AIDS and bioterrorism have affected regulatory policy in recent years. The author reviews FDA's changes in policy and philosophy during the past 30 years.