
The international pharmaceutical supply-chain consortium Rx-360 held a launch meeting in Europe last week with more than 135 industry and regulatory representatives in attendance.

The international pharmaceutical supply-chain consortium Rx-360 held a launch meeting in Europe last week with more than 135 industry and regulatory representatives in attendance.

Company and People Notes: Genzyme provides updates on enzyme replacement products and FDA complete response letter; Watson appoints VP of global operations; more...

On Sunday, Nov. 8, 2009, the Pharmaceutical Research and Manufacturers of America (PhRMA) expressed its disappointment with the US House of Representatives's healthcare-reform bill.

The European Medicines Agency, the European Center for Disease Prevention and Control, and the Heads of Medicines Agencies issued a European strategy for H1N1 vaccine benefit–risk monitoring.

Also, FDA and WebMD increase collaboration and PPD is awarded contract to evaluate the agency's postmarket spontaneous-adverse-event surveillance system.

The Society of Chemical Manufacturers and Affiliates (SOCMA) reported last week that the US Food and Drug Administration responded to the association's citizen petition relating to the inspection process of foreign drug-manufacturing facilities.

In response to a request from the US Food and Drug Administration, the US Pharmacopeial Convention (USP) revised its standards for propylene glycol and sorbitol solution

Company and People Notes: sanofi aventis forms pact with Micromet; Laureate Pharma adds members to its business team.

Last week, the steering committee and expert working groups of the International Conference on Harmonization (ICH) met in St. Louis, Missouri. A major success of the meeting was the adoption of several annexes to ICH Q4B on pharmacopeial texts.

A panel of industry and regulatory experts, including FDA, discuss efforts to secure the global pharmaceutical supply chain. This article contains bonus online-exclusive material.

New tests solve one issue, but cheaper plastic and new stoppers cause problems.
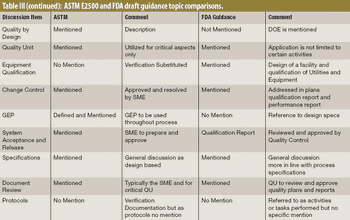
The author explores differences between two qualification documents, the draft guidance from FDA "Process Validation: General Principles and Practice" and the ASTM E2500-7 standard "Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment."

The heightened focus on risk raises concerns about delays in approving new drugs.

With a five-yar revision cycle around the corner, USP will hit or miss the collaboration mark.

While Congress debates hundreds of healthcare plan proposals, perhaps we, the public, can get in the game too.

New standards may overlook critical qualification needs.

New pricing controls and healthcare reforms may be pushing the pharmaceutical market out of this southeast Asian country. This article contains bonus online-exclusive material.

The authors used a light-transmission-based static division system to detect particles of foreign contaminants in prefilled vials.

This article provides an update to the author's Viewpoint column, "Changes to Vial Labels May Affect Patient Safety," which ran in Pharmaceutical Technology's March 2009 issue.

Mark Copley discusses the methods used for DPI testing and the challenges presented by the current regulatory framework.

With counterfeit drugs on the rise, the Chairman and CEO of Ahura Scientific discusses the issue and explains how a handheld device is helping companies to tackle the problem.

The House Energy and Commerce Committee approved H.R. 2868, the "Chemical Facility Anti-Terrorism Act of 2009," a measure to modify and codify existing requirements of the Chemical Facility Anti-Terrorism Standards (CFATS) program.

Also, FDA's DDMAC issues Warning Letter to King Pharmaceuticals; draft guidance released for SPL standards for content of labeling.

The US Food and Drug Administration needs to enhance its oversight of drugs approved on the basis of surrogate endpoints, according to a September report from the Government Accountability Office (GAO) that was released this week.

New barcoding technologies are helping to aid product traceability and fight counterfeits. Mark Beauchamp of Citizen Systems Europe explains how.