
The US Food and Drug Administration plans to hold a public hearing to discuss the promotion of agency-regulated drugs and medical devices on the Internet and using social media tools, according to a notice in the Sept. 21, 2009, Federal Register.

The US Food and Drug Administration plans to hold a public hearing to discuss the promotion of agency-regulated drugs and medical devices on the Internet and using social media tools, according to a notice in the Sept. 21, 2009, Federal Register.

Also, FDA extended the deadline for the pilot program for the submission of CMC information.

The US Food and Drug Administration sent Bayer Schering Pharma (Berlin) a Warning Letter on Aug. 5, 2009, citing deviations from current good manufacturing practice in the manufacture of nonsterile active pharmaceutical ingredients (APIs).

Product failure can be potentially fatal to a customer and have severe implications for manufacturers; however, many pharma companies still believe that complaint management is not related to the overall management of customer relationships.

The Senate Finance Committee through its chairman, Max Baucus (D-MT), introduced on Sept. 16, 2009, a bill, "America's Healthy Future Act of 2009," representing the latest Congressional proposal for healthcare reform.

Also, FDA's enforcement of its rules for response times for Form 483s went into effect this week, more...

Speaking to the audience of the 2009 PDA-FDA Joint Regulatory Conference in Washington, DC, this week, US Food and Drug Administration Principal Deputy Commissioner outlined four principles that define FDA's role as a public health agency.

Company and People Notes: Sanofi Pasteur signs H1N1 vaccine deal with Brazilian government; Helsinn Group appoints CEO of US business; more...

Investing time and money in auditing and optimizing a steam system can pay off quickly, especially because the costs of energy, maintenance, and downtime are steadily rising.

Generic-drug companies are increasingly viewing the development of controlled-release formulations as a way of obtaining a competitive edge, according to a report published by Espicom Business Intelligence in late August 2009.

The US Pharmacopeial Convention (USP) and the Permanent Commission of the Pharmacopeia of the United Mexican States (FEUM) signed a memorandum of understanding (MOU) last week.

Also, Orexo and Novartis form agreement; Affitech appoints Robert Burns CEO; more...

The US Food and Drug Administration last week issued the final draft of its guidance for industry titled Labeling of Nonprescription Human Drug Products Marketed Without an Approved Application as Required by the Dietary Supplement and Nonprescription Drug Consumer Protection Act: Questions and Answers.

Implementing Europe's Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) program, will require a massive increase in animal testing and cost six times more than previously estimated.

Also, FDA issues several recent enforcement letters to Cambrex, Johnson & Johnson, and Pedinol.

Later this month, Dr. Barbara Jentges, the managing director at PhACT GmbH, a regulatory consulting and training firm based in Duggingen, Switzerland, will be speaking at the 2nd Vetter Drug Management Leadership Conference in Germany.

Thanks to their keen observations, these auditors reveal the true culprits of deviations.

After years of promomting QbD concepts, FDA's ready to take action on nonconformers.

Robotic systems provide flexibility and efficiency (and they're not as difficult to use as you think). This article contains bonus online-exclusive material.

Authenticating tools help identify counterfeit drug products. This article contains bonus online-exclusive material.
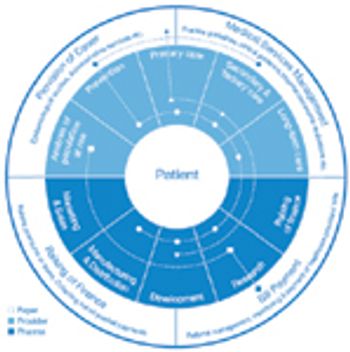
Personalized medicine and integrated healthcare delivery require new business and pricing models. This article contains bonus online-exclusive material.

Health crises generate support for new vaccines and treatments for diseases found in developing nations.

Representatives of Japan's MHLW report on recent ICH activities and what the ministry expects from Q11.

As new process validation guidelines emerge, industry needs to reinvent how it releases product.

IPEC's new stability testing guide takes into account the full supply chain's storage conditions.