
In January 2011, the European Medicines Agency (EMA) announced the new revision of EudraLex Volume 4 (GMP) - Annex 11 'Computerised Systems', and the consequential amendment of EudraLex Volume 4 - Chapter 4 ?Documentation?.

In January 2011, the European Medicines Agency (EMA) announced the new revision of EudraLex Volume 4 (GMP) - Annex 11 'Computerised Systems', and the consequential amendment of EudraLex Volume 4 - Chapter 4 ?Documentation?.

Following recent accusations that the EMA puts companies' commercial interests ahead of public safety, the EMA has responded by emphasizing recent initiatives at the agency to improve data access.

FDA held a joint meeting of its Nonprescription Drugs Advisory Committee and Pediatric Advisory Committee to discuss whether new dosing information for oral over-the-counter drug products containing acetaminophen should be added to the label for children under age 2.

Teva Pharmaceutical Industries agreed to pay shareholders $460 million in cash to acquire a 57% stake in Taiyo Pharmaceutical Industry. Teva also will offer to buy all outstanding shares of Taiyo.

Johnson & Johnson subsidiary Janssen-Cilag International reported that the company is working with regulatory authorities in five countries to address trace amounts of 2,4,6- tribromoanisole identified in five batches of the HIV/AIDS medicine Prezista.

EMA is working with its European and international regulatory partners to monitor and evaluate ?the possible risk of radioactive contamination of medicines manufactured in Japan following the radiation leak from the Fukushima Daiichi nuclear power plant.?

Regulatory approvals for new biopharmaceuticals in the United States have nearly doubled in the past decade compared with the 1990s, says a Tufts study.

How do you assign a minimum sample weight for a US Pharmacopeia <41> balance application when the tested repeatability gives a standard deviation of zero?

FDA issued a new guidance for industry concerning the submission of summary bioequivalence data for abbreviated new drug applications.

FDA is asking for input on the development of a user-fee program for biosimilar and interchangeable biological product applications.

The IPEC is soliciting public comment about a draft plan for the independent certification of manufacturers and suppliers of pharmaceutical excipients.

FDA is collecting public comments on a series of studies that the agency plans to conduct on online direct-to-consumer promotion of prescription drug products, according to an announcement in the Federal Register.

Eastern Europe's pharmaceutical leader, Hungary, is working to maintain its number-one status while also pursuing new avenues, especially in biopharmaceuticals.

Regulators and standard-setting bodies are re-examining over-the-counter drugs.
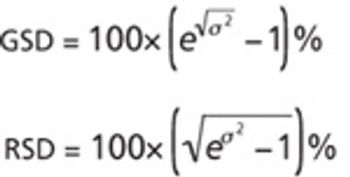
The author argues that traditional concerns about repeatability and intermediate precision remain valid but insufficient.

Those who doubt there's faith in science, should check out our annual Bioprocessing Survey.

FDA, NIH and industry seek new strategies to spur drug development and promote access to therapies.

Monograph modernization and standards donation go hand in hand.

India has the potential to become the new star of the biotechnology industry.

Many factors affect research results.
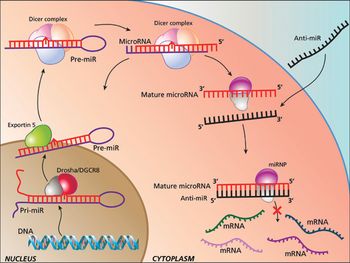
The authors provide further insight into microRNA biology, and the simplicity of anti-miR oligonucleotide drug delivery.

The authors question certain aspects of the industry's current regulatory-compliance strategy and suggest that aseptic-process control and evaluation should be revised.

The author describes recent developments to help overcome the downstream processing bottleneck. This article is part of a special issue on Sterile Manufacturing and Bioprocessing.

Linking peptides to polyethylene glycol, or PEGylation, has helped improve pharmaceutical therapeutics in several ways. A wave of new techniques is now ushering in further advances.

FDA has released a list of its strategic priorities for the next five years to address new global challenges.