
A summary of the latest steering committee and expert working groups' meeting.

A summary of the latest steering committee and expert working groups' meeting.

Might European officials reverse their position on acceptable production methods?

International Federation of Pharmaceutical Manufacturers and Associations takes global action to improve public health.

Industry buy-in is increasing as pharma companies proceed with select projects and research.

After a series of structural changes, the author wonders whether USP is undergoing an identity crisis. USP CEO Roger L. Williams responds.
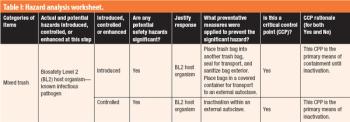
The third in a series of eight case studies from the Product Quality Research Institute focuses on facility biocontainment and inactivation.
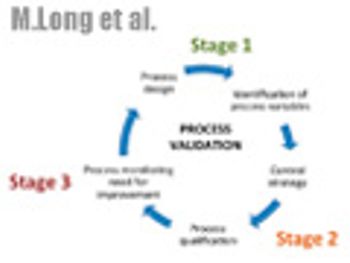
The authors desribe the three-stage approach to validation that is outlined in the new guidance and discuss questions surrounding implementation.

By providing an information "fingerprint", infrared red spectroscopy is a useful tool for identifying counterfeit pharmaceuticals. The authors identify best practices for ensuring compliance.

Clarifying GMPs for excipients used as actives.

A Q&A with Brian Johnson, senior director of supply chain security at Pfizer, moderated by Patricia Van Arnum. Part of a special Ingredients issue.

USP and the Brazilian National Agency of Sanitary Surveillance are teaming up to develop joint education activities for professionals involved in formulating and using pharmacopeial monograph standards in Brazil.

FDA published a draft guidance that lists recommendations to follow, data to provide, and criteria to meet and describe in new drug applications and abbreviated new drug applications for scored tablets.

EMA announced that a new version of the validation criteria for electronic applications for human medicines comes into effect on Sept. 1, 2011.

African Community Joins ICH's Steering Committee.

The author examines sample-preparations methods used in inductively coupled plasma–optimal emission spectroscopy for four test metals.

Last week, Ben Venue Laboratories decided to exit the contract-manufacturing business during the next several years, thus ending more than 70 years of service in this field. To ensure the supply of medically necessary products, the company will work with its customers to develop and execute long-term transition plans.

FDA, Arkansas Sign Agreement to Enhance Regulatory Science.

Since China's State Food and Drug Administration revised its GMPs last year, the agency has been making other improvements as well to improve the quality of its pharmaceutical manufacturing industry.

We try to use previous experience to develop the optimal granulation process for each new ingredient, but we sometimes get it wrong. Many times, we?ve taken materials from the granulator only to find that they exhibit poor flow and insufficient density. How can we easily determine the end point of a given granulation process?

Achieving a consistent level of quality control could greatly reduce waste and save money for the pharmaceutical industry. But why has talk about Six Sigma died down at a time when it could be of great benefit?

FDA Issues Final Guidance Regarding cGMPs for PET Drugs.

EMA has concluded in a recent report that a pilot program investigating the mutual benefits of joint international inspections of API manufacturing facilities has been a success. FDA has also reached a similar conclusion based on the findings.

The Society for Chemical Manufacturers & Affiliates commented on EPA's modifications to the Inventory Update Reporting rule, also broadly know as the Chemical Data Reporting rule. EPA issued the final rule earlier this month.

Since the passage of the BPCI Act in 2009, manufacturers have been waiting for guidance from FDA on what that approval process will look like.

The International Society for Pharmaceutical Engineering published a guidance document that defines current best practices in pharmaceutical manufacturing applications for handling gases that come into direct contact with the biopharmaceutical and pharmaceutical process steams.