
The Laboratory Services Division of the Philippine Food and Drug Administration (FDA) has attained internationally recognized accreditation for its testing and calibration laboratories, according to a July 12, US Pharmacopeia (USP) announcement.

The Laboratory Services Division of the Philippine Food and Drug Administration (FDA) has attained internationally recognized accreditation for its testing and calibration laboratories, according to a July 12, US Pharmacopeia (USP) announcement.

A history of the selection of the widely used significance level leaves much to be desired.

The 2D Data Matrix barcode is a familiar sight for most pharmaceutical manufacturers as printing is implemented to meet certain regulations.

A method for determining sample size is finally getting some respect.


Liquids are used in a wide variety of industries, either during manufacture or as end-products in themselves. Whatever the end product, quality control of liquids is crucial to many industries.

Leading experts share insight on the current and future direction of process analytical technology. This article contains bonus online material.

Grace McNally, in the Office of Compliance at FDA's CDER, discusses the role of QbD and pharmaceutical quality systems as it relates to contract manufacturing.

The quality assurance environment is forcing pharmaceutical companies to face new challenges. In light of this, the authors conducted a study of professionals from some of the world's top pharmaceutical companies to identify key QA concerns.

Is the square root of (N) + 1 a statistically valid scheme?

The author of an ambitious book about quality control falls short of reaching his goals.

A book guides readers through the regulatory requirements for computerized quality systems.

Myth versus Reality: What does Q10 implementation really mean for my company?

A new book explains process analytical technology, drug stability, and quality.

Process designs and control strategies can be improved by adopting a risk-based approach to product quality. The author describes how this approach can be applied to spray-drying operations.

QbD, PAT, design space, what's it all about? This seems to be a common industry response to FDA's directional push.

Here's a look at some of the most interesting and thematic responses you-our readers-provided for Pharmaceutical Technology's anniversary survey of industry advances and directions.

Signature authentication technology has evolved to become a stong tool for improving confidence in verification.

San Juan, PR (Apr. 25)-Regulatory and quality issues were prominent discussion topics at this year?s ExcipientFest conference and exhibition.
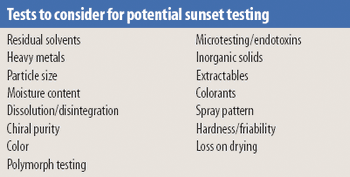
The concept of Acceptable Analytical Practices (AAPs) was developed by the Analytical Technical Group of the Pharmaceutical Research and Manufacturers of America to share information about how the pharmaceutical industry has implemented chemistry, manufacturing, and controls and quality guidances of the International Conference on Harmonization and worldwide regulatory authorities. The AAP process identifies and addresses critical issues in which guidance is lacking, ambiguous, or contradictory. AAPs were designed to provide a forum where one could learn from the experience of experts in pharmaceutical analysis and enhance the understanding of analytical practices that reflect good science and sound regulatory compliance. This article summarizes the discussion points from a meeting regarding the Justification of Specifications topic.
This article considers the distinction among the terms qualification, validation, and verification in the context of pharmacopeial usage.A recommendation for a standardized usage of the terms validation and verification is provided,and general requirements for validation and verification activities are given.The article also emphasizes the importance of knowing when validation or verification is necessary relative to the use of a method to satisfy pharmacopeial article requirements (for which a monograph exists in the pharmacopeia) or for nonpharmacopeial use.

The authors describe the methods by which precise analyses of stable-isotopic abundances can be used in security and forensic applications for pharmaceutical materials. These methods include product and process authentication of raw materials, pharmaceutical intermediates, drug substances, formulated drug products, and synthetic pathways. Collectively, these methods can be used to investigate and mitigate patent infringement. In the future, more complete examples will be presented containing full isotopic results and the application of the methods described in this article.

As technology for impurity analysis improves, scientists are gaining better information and asking for more regulatory guidance.
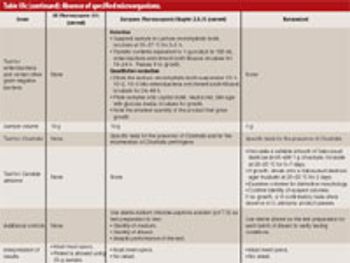
The US Pharmacopeia (USP), the Japanese Pharmacopoeia, and the European Pharmacopoeia "Microbial Limits Tests" are in the final stages of harmonization. The harmonized USP chapters are slated for implementation in 2009. This article describes the harmonized USP Chapters <61> "Microbial Enumeration," <62> "Absence of Specified Microorganisms," and <1111> "Microbiological Attributes of Nonsterile Pharmaceutical Products," and suggests they will likely require some revalidation of existing methodologies. Companies should put plans in place immediately for this work and show consistent progress towards this goal.

We really knew what we were doing-until we opened the column for a routine repacking.