
Representatives of contract service organizations that develop biologic-based drugs discussed technology trends such as high-throughput screening and single-use systems.

Representatives of contract service organizations that develop biologic-based drugs discussed technology trends such as high-throughput screening and single-use systems.

Experts from contract testing laboratories and service organizations shared their perceptions of analytical testing advances, and challenges still ahead.

Contract services ride high as funding floods bio/pharma.

Representatives of contract service organizations that specialize in parenteral drug development and manufacturing describe the evolving trends.

Despite the growth of specialist companies with capabilities across various therapeutic areas in Europe, there is still a need for early development expertise with end-to-end pharmaceutical manufacturing capabilities.

Contract development and manufacturing organizations identify trends, challenges, and emerging technology and service needs for solid and semi-solid dosage forms.

For a bio/pharma industry in flux, contract services are playing a greater-and more diverse-role in drug development.

Packaging Coordinators, Inc. expands its services with acquisition of Penn Pharmaceutical Services.

Hovione will use Merrion's GIPET absorption-enhancing technology for solid-dosage drugs.

As payers refuse to cover new drugs, CMOs take a hit.

PharmSource special report shows demand for cytotoxic injectable drugs could tap existing CMO capacity.
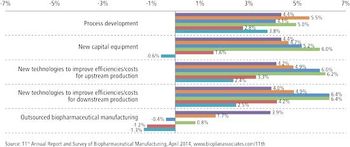
With budgets growing, clients see CMOs' costs as less crucial.

Paul Nelles of Vetter Development Service discusses prefilled syringes.

DCAT's Sharp Sourcing 2014 educational program offers pharmaceutical and biopharmaceutical companies a forum to gain best practice insights in sourcing and procurement.

Industry experts discuss how extractables and leachables studies are designed using a risk-based approach.

Data integrity in the analytical laboratory is an area of increasing focus for regulators such as FDA.

The CMO industry's value proposition is limiting its market penetration.

Biopharma companies should not overlook India's growing market.

The international pharmaceutical industry is changing its approach to R&D and is increasingly relying on outsourcing for drug discovery.

Outsourcing activity remains strong and unlikely to abate, especially in more traditional areas.

Changes in company ownership shake up the CMO industry.

Thirteen companies are accepted for participation in the supply chain program.

CMOs may find opportunities in alternative expression services.

Contract manufacturing organizations that are established in key markets can provide a competitive edge.
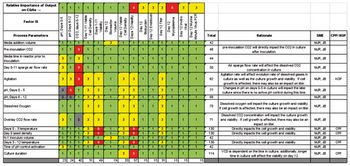
Traditional project decision-making is compared with a QbD approach.