
A Q&A with Erik van den Berg, CEO of AM-Pharma, on recent industry trends.

A Q&A with Erik van den Berg, CEO of AM-Pharma, on recent industry trends.

China's drug-distribution network has been a mess for years, but government reforms and industry focus are unveiling new opportunities for market order and growth.

Brazil's generic-drug market is growing steadily.

The evolving bio/pharmaceutical business model poses risk for CMOs.

More collaboration and expanded oversight aim to compel manufacturers to follow GMPs.

Pharma announces plans for the year ahead at annual JPMorgan Global Healthcare conference.

As biopharmaceutical development and commercialization increases, companies are expanding their cold-chain capabilities.
Industry optimism is on the rise for 2012.

The Asian nation is strategizing to take the lead over its regional competitors in pharmaceutical exports.

A technical forum featuring Catalent Pharma Solutions, SAFC, and Neuland Laboratories.

The author describes an equation that can be used to define the Quality relationship between a contract manufacturing organization and a client, including how to factor in both party's needs and regulatory commitments.
The author examines recent examples of preferred-provider collaborations.
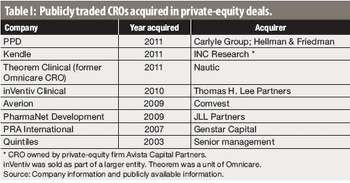
Some recent private-equity buyouts of CROs show both the upside and downside for investors.

Each developing economy has unique economic, political, and cultural issues that help define its pharmaceutical market. To succeed, multinational pharmaceutical companies will have to adapt differently.

The author examines the opportunities and positioning of contract service providers.

Pressure to approve new user fees opens the door to action on drug shortages, prices, and regulation.

As part of the BRIC bloc with Russia, India, and China, Brazil is one of the world's leading emerging economies and is also considered by IMS Health to be one of seven pharmerging nations, which also include Mexico, Turkey, and South Korea.

Some recent private-equity buyouts of CROs show both the upside and downside for investors.

The European Union market takes steps toward continuous processing and modular facilities.

A Q&A with Gilles Cottier, president of SAFC, on recent industry trends.
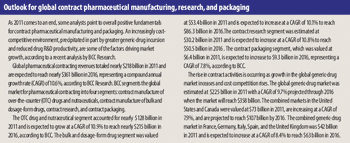
Expansion activity was limited as fine-chemical producers and CMOs of API and intermediates grapple with changing industry fundamentals.

A dearth of late-stage candidates could hurt the pharmaceutical services market in the future.

Cleanliness is crucial, even if zapping and trapping is necessary to reduce product contamination.

Added responsibilities and outside concerns prompt overhaul of agency's structure.

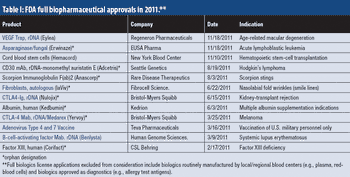
Drug shortages, supply-chain security, generic-drug incursion, and flexible manufacturing models are some of the issues shaping the bio/pharma industry.