
BeiGene is set to build late-stage clinical and commercial production capacity for cancer monoclonal antibodies with GE Healthcare’s KUBio, the prefabricated biopharma facility based on single-use technologies.

BeiGene is set to build late-stage clinical and commercial production capacity for cancer monoclonal antibodies with GE Healthcare’s KUBio, the prefabricated biopharma facility based on single-use technologies.

Evonik completed a EUR 36 million expansion of its contract-manufacturing capabilities for API and advanced intermediates in the United States and Germany.

The $425-million acquisition adds formulation development and finished dosage manufacturing capabilities to Cambrex’s existing global API manufacturing network.

Sharing know-how can help resolve common bio/pharma technical challenges.

Partnerships, mergers, and new services indicate that biologics are continuing to influence CMOs’ and CDMOs’ decisions to expand their biopharmaceutical services.

Alcami and the University of North Carolina Wilmington received a grant from the National Institute for Innovation in Manufacturing Biopharmaceuticals to prepare students for careers in pharmaceutical sciences.

The acquisition strengthens Nelson Labs’ outsourced testing capabilities for the pharmaceutical and medical device industries.

The company is certified as a manufacturer of pressure vessels and components for use in China.

An expansion of laboratory facilities in Geneva, Switzerland expands SGS services for high-order structure analysis.

The new Testa Center in Uppsala, Sweden is a collaborative test bed offering biotechnology equipment from GE Healthcare for process development.

Drug product approval from FDA follows previous approvals from European and Japanese authorities.

Industry experts discuss the challenges of performing glycan analysis and how companies can gain specific expertise from outsourcing partners.

API can be mixed with silicone and other polymers to create drug-delivery combination products.

CMOs have been active over the past year in expanding their biologics production and capabilities.

This article provides a sampling of the latest investments, expansions, and acquisitions by small-molecule contract service providers.

Heightened uncertainty means CDMO executives need to play out planning scenarios.

Why shouldn’t biopharmaceutical manufacturers be able to leverage standardized construction practices to improve time to market?
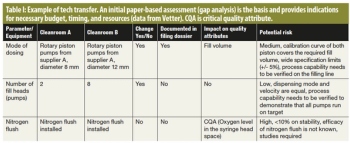
Real-life examples illustrate how to reduce the risks for each transferring partner and ensure that the development process meets regulatory requirements.

Quartzy, an online laboratory supply management company, will offer lab products from Bioline, Biotium, and MP Biomedicals.

The API and drug product provider will invest £375,000 (US$493,000) in additional nuclear magnetic resonance (NMR) instrumentation at its headquarters in Craigavon, UK.

The glass and chemical provider will expand its synthetic pharmaceutical intermediate and API production capacity at its plant in Chiba, Japan.

The acquisition will place Cambrex into the finished dosage form CDMO market.

CELLforCURE will produce cancer CAR-T treatments for Novartis at a manufacturing facility in Les Ulis (Essonne), France.

Catalent’s acquisition of Juniper Pharmaceuticals further expands its early development capabilities.

The company expanded its extended workbench laboratory services program to support the ongoing manufacturing and development of Flexion Therapeutics’s Zilretta (triamcinolone acetonide extended-release injectable suspension).