
HHS Awards $97-Million Vaccine Development Contract

HHS Awards $97-Million Vaccine Development Contract

Interphex new

The authors propose a strategy for classifying and validating inprocess testing methods.

Managers of FDA-regulated firms must be proactive in how they manage their company?s compliance with good manufacturing practices regulations.

The author proposes a new analytical graphic, the sector chart, which presents data that cannot be adequately presented with current graphs.This chart combines features of zone charts with the basic principle of precontrol charts. It addresses engineering control and is superior for representing data such as beneficial and adverse trends.

The validation of alternative microbiological testing is an opportunity for a manufacturer to decrease the amount of time required for laboratory results.To properly validate these alternatives, a practitioner must first identify what is being studied. The regulatory effect on established product and process specifications and levels must be completely evaluated, as changing the method of analysis may well change the pparent number in the sample.

The C-SOC team must develop a network of committed industrial partners who will participate in the direction, execution, and evaluation of research and educational activities.

RFID and battery-powered containers are among the developments receiving attention in the pharmaceutical cold chain.

The principles of direct compression haven’t changed in more than a century. So why can the topic still pack a ballroom?

X-ray microtomography has great potential for improving the understanding of the structural features of solid dosage forms and the changes in those features during manufacturing, handling, and storage.This article describes the basic principles of the technique and provides examples of its potential applications.

Achieving the promised benefits of offshore sourcing to India and China is more complicated than it seems.

The sterility testing of samples from an aseptic process may be considered an entirely separate aseptic process that is subject to the same types of adventitious contamination as the aseptic process itself.

An increasing number of new compounds are being introduced into pharmaceutical pilot plants.The knowledge base for these compounds regarding their toxicities,physical handling, and cleaning is limited.The authors examine various approaches for addressing the cleaning validation of new compounds and discuss the role of determining appropriate visible residue limits.

A comparative study of three air samplers used for bioaerosol collection was performed to evaluate the average recovery of colony-forming units and assess the precision of each device.

To meet the requirements of the USP ^755& Minimum Fill and ^698& Deliverable Volume tests, target fill levels greater than 100% must be established.This article proposes a criterion for establishing an appropriate target fill level such that a sample will have a 95% probability of passing these USP tests at 95% confidence.

The authors argue that chlorine dioxide (CD) is a safe and effective decontaminating agent that can be used for challenging applications.The effectiveness of CD gas for sterilizing complex isolator systems is studied.

The authors study the effects of sweet potato and cocoyam starches on the compressional and mechanical characteristics of a paracetamol tablet formulation using cornstarch BP.

Innovators and generics makers are staking out positions on follow-on proteins and other manufacturing issues.


Managers of FDA-regulated firms must be proactive in how they manage their company's compliance with good manufacturing practices regulations.

This article looks at the different approaches to patenting in Europe and the US...
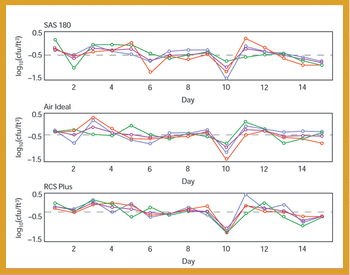
A comparative study of three air samplers used for bioaerosol collection was performed to evaluate the average recovery of colony-forming units and to assess the precision of each device.
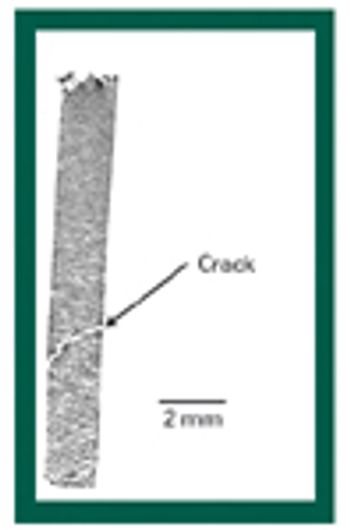
X-ray microtomography has great potential for improving the understanding of the structural features of solid dosage forms and the changes in those features during manufacturing, handling, and storage. This article describes the basic principles of the technique and provides examples of its potential applications.
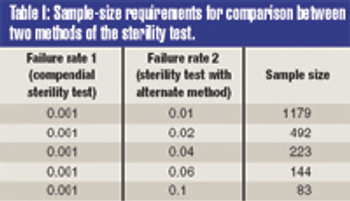
The validation of alternative microbiological testing is an opportunity for a manufacturer to decrease the amount of time required for laboratory results. To properly validate these alternatives, a practitioner must first identify what is being studied. The regulatory effect on established product and process specifications and levels must be completely evaluated, as changing the method of analysis may well change the apparent number in the sample.
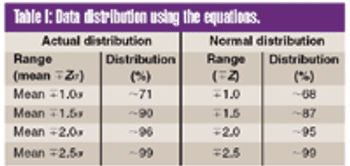
To meet the requirements of the USP ?755? Minimum Fill and ?698? Deliverable Volume tests, target fill levels greater than 100% must be established. This article proposes a criterion for establishing an appropriate target fill level such that a sample will have a 95% probability of passing these USP tests at 95% confidence.