
With the acquisition of Medipac’s tube filling assets for effervescent tablets, Romaco can now offer the complete line configurations for effervescent tablets.

With the acquisition of Medipac’s tube filling assets for effervescent tablets, Romaco can now offer the complete line configurations for effervescent tablets.

Pharmaceutical manufacturing facilities can help prevent contamination and cross contamination by using color coding.

The University of Sheffield has appointed Cobra Biologics to advance novel fusion protein technology into Phase 1 clinical trials.
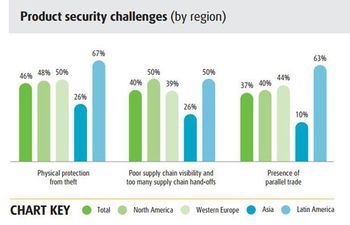
UPS’ 2015 “Pain in the Chain” survey suggests that pharma companies are getting better at product protection, cold chain and regulatory compliance, but need to improve cost control and planning for unexpected events. Lack of transparency and “too many handoffs” remain major challenges.

Expansions at Catalent’s Kansas City, MO, and Madison, WI facilities made in response to industry demand.

Baxter expands capacity for lyophilized cytotoxic oncology therapies at its fill/finish facility in Halle, Germany.

Integrated pharmaceutical blister-packaging equipment systems strengthen serialization and brand protection capabilities.
Dynamic powder testing and measurement of bulk powder properties can complement shear cell testing to identify the causes of poor hopper performance in solid-dosage drug manufacturing.

Experts at the ISPE annual meeting describe best practices, including containment and production in classified spaces.

Operations at Catalent’s Beinheim, France, softgel facility were suspended following suspected deliberate action to misplace capsules.

GE Healthcare Life Sciences and Emerson Process Management collaborate in biopharmaceutical manufacturing processes.

Roche will invest in Switzerland but leave sites in Ireland, Spain, Italy, and the US as it focuses on lower-volume, specialized medicines.

Applications in the Rockwell Software Studio 5000 development environment add functionality for automation engineers.

Under the terms of the agreement, Daewoong will annually purchase minimum API quantities of erdosteine of approximately EUR 25 million from Edmond Pharma, a Recipharm Group company.

The UK’s National Biologics Manufacturing Centre will use Novasep’s BioSC Lab for protein purification.

AstraZeneca’s solid-dose facility in Taizhou, China received ISPE’s FOYA 2015 Overall Winner award.

Industry veteran Mark Kontny joins board of directors at Grand River Aseptic Manufacturing.

This investment comes in response to the increasing demand for high quality and safe pharmaceutical packaging. rlc already has two pharma packaging centers in Rüdersdorf near Berlin, Germany and in Poznan, Poland.

GE Healthcare's KUBio prebuilt modules were shipped from Germany to JHL Biotech in Wuhan, China.

Biotech boom, niche markets, smaller batch sizes and high potency manufacturing are among the key trends shaping the pharmaceutical industry of the 21st century, according to Christian Treitel from Bosch Packaging Technology.

The latest revisions to the international pharmacopoeia standards for glass pharmaceutical packaging have emphasized the importance of assessing delamination propensity of pharmaceutical glassware, including bottles, vials, cartridges, and syringes.

South Africa’s Biovac Institute, which develops and produces vaccines for the country, launched a public-private partnership with Pfizer to enable local manufacturing of Prevenar 13, a vaccine against pneumonia-causing bacteria.

Problems in an induction-sealing process, such as untorqued or crooked caps, can be identified and corrected in real time using dynamic thermal imaging.
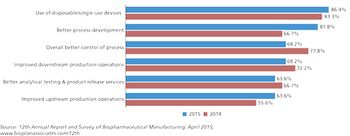
Better process development is creating industry benchmarks for bioprocessing.
Delamination of glass packaging is a source of particulates in parenteral drugs, but identifying the root cause allowed the design of an improved manufacturing process for glass vials.