
Almac partnered with Optel Vision to provide a line-level solution (hardware and software) integrated with Almac’s proprietary level three, site-level software.

Almac partnered with Optel Vision to provide a line-level solution (hardware and software) integrated with Almac’s proprietary level three, site-level software.

The company announced the launch of its first-in-class Lynx CDR connectors at INTERPHEX 2016.

Highly potent or cytotoxic drugs require special handling

The Subcommittee for Advanced Manufacturing of the National Science and Technology Council highlights biopharmaceutical manufacturing as an emerging priority.

Packaging innovations displayed at INTERPHEX include advanced materials, inspection systems, and filling systems.

Traditional methods of measuring the effectiveness of vaccines against the flu are called into question by new findings from the NIH.

Sanofi will invest in its Geel, Belgium facility in order to support its pipeline of monoclonal antibodies.

The National Institute of Health will conduct an internal review of the National Cancer Institute’s cell manufacturing facilities, which will affect multiple Kite projects.

By focusing on laying strong technology foundations, pharmaceutical businesses can make informed decisions, equip themselves with the capabilities to eliminate threats, and future-proof operations.

GE Healthcare Life Sciences has expanded production capabilities at its Pasching, Austria facility for sterile liquids.

FDA approved an update in the manufacturing of Prezista (darunavir) using a continuous manufacturing line at Janssen Supply Chain’s facility in Puerto Rico.

The FDA guidance describes information to provide to the agency when submitting a proposed proprietary name.

A report by Reuters found that four of the top 10 most widely used drugs increased 100% since 2011, while the other six increased 60%.

The agency has announced the creation of the Combination Products Policy Council to address issues related to combination products.

Texture analyzers can be used to evaluate wall hardness and elasticity of softgel capsules.

Non-destructive container closure integrity testing allows 100% inspection of all ampoules, syringes, vials, and cartridges.

Consider the 3 Ps (product, process, and patient) when choosing a parenteral packaging material.
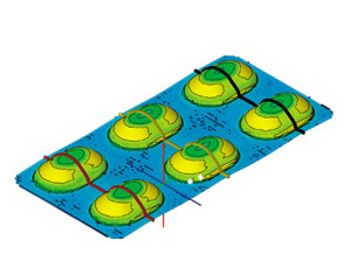
Science-based software and testing services expedite the material selection process and ensure blister packs deliver adequate barrier protection for solid-dosage forms without over-packaging.

Transferring the manufacturing of a drug from one scale to another or between manufacturing sites presents both technical and business challenges.

Susan Schniepp, distinguished fellow, and Andrew Harrison, chief regulatory affairs officer and general counsel, both of Regulatory Compliance Associates, discuss requirements for successful product technology transfer.

Pharmaceutical Technology spoke with Bill Randolph, vice-president, Technical Services, Janssen Supply Chain, about some of the considerations for technology transfer of a continuous, solid-dosage manufacturing process and what he sees as the outlook for continuous manufacturing.
Manufacturing highly toxic compounds in a biopharmaceutical environment tests equipment and systems.
Choosing the right container and container closure system is crucial for ensuring product quality, safety, and efficacy of a biologic formulation.

Headspace moisture analysis is a rapid non-destructive analytical method that may potentially address the limitations of traditional methods used for residual moisture determination.

The authors discuss the concept design of a versatile, sustainable, small-scale facility in Malta that conforms to the European Union’s current good manufacturing practices.