
The therapeutic candidate AZD8601is an investigational mRNA-based therapy that will be tested for its ability to regulate the protein that influences vascular tissue growth.

The therapeutic candidate AZD8601is an investigational mRNA-based therapy that will be tested for its ability to regulate the protein that influences vascular tissue growth.

The vaccine candidate has also won a Priority Medicines (PRIME) status from EMA.


Zenith appointed Carlos Machado as serialization director to lead the company’s sales, operations, and post project support services.

The company manufactures biological drug products and intermediates for the allergy vaccine market.

The company voluntarily recalls all lots of lyophilized HCG and sermorelin.

GenPak Solutions is cited by FDA for cGMP violations at its Hilliard, Ohio facility.


Gamma-stable fluoropolymers are an alternative material for single-use bags and assemblies in biopharmaceutical manufacturing.

The company plans to purchase and develop an 18-acre site in Des Plaines, Illinois.

The collaboration will focus on the investigational candidate JTX-2011 and up to four other early-stage programs in immune-oncology.

The company invested in the MicroSeq Rapid Microbial Identification System from Applied Biosystems at its Illinois facility.

The antisense drug will be the first in the companies’ joint development deal for medications to treat autoimmune disorders of the gastrointestinal tract.

FDA issued a warning letter to the Worthing, UK facility for cross contamination and microbial contamination cGMP violations.

GE further ramps up its cellular and gene therapy offerings with its acquisition of Biosafe, a cell processing solutions company.

The company will invest €100 million in the expansion of its Athlone facility.

Novo Nordisk broke ground on an expanded production plant for insulin in Kalundborg, Denmark.

Teligent is expanding its manufacturing and R&D complex in New Jersey.

A study shows high levels of ADAs to infliximab at the beginning of treatment were associated with a poor response later on.
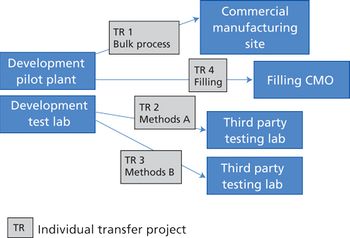
Integrating quality and compliance with technology transfer and careful project management are key in starting up a facility and launching a biologic drug.

Innovations protect product quality, improve productivity, enhance sustainability, and simplify usage for patients and caregivers.

Designing systems using the principles of good documentation practice, including validated audit trails, is a key piece of a manufacturing data integrity program.

Early planning for the integration of clean-in-place systems for equipment cleaning is key.

CPhI Pharma Awards seek nominations for excellence in development and manufacturing.

Aseptic spray drying provides an alternative to lyophilization as an enabling stabilization technology for parenteral biologic formulations.