
Recipharm adds hardware, software, and cloud services for serialization compliance processes.

Recipharm adds hardware, software, and cloud services for serialization compliance processes.

MilliporeSigma and the International Vaccine Institute in Seoul, South Korea aim to develop more robust, scalable vaccine manufacturing processes.

Studying broadly neutralizing antibodies in infants may lead to new pathways in HIV vaccine development.

The Pharmacopoeial Discussion Group approved monographs and plans to harmonize several others.

The agency cited the company for sterile manufacturing violations.

A research team associated with Dr. Carl June announces it has discovered a way to engineer a patient’s own immune cells to recognize cancer-specific glycoantigens on tumor cells.

Pfizer broke ground at its Andover, Massachusetts campus on a clinical manufacturing facility for complex biologics and vaccines.
Computerized systems can solve some of the data integrity problems with conventional paper-based systems.

Results of a Phase II clinical trial reveal that stem-cell transplantation treatment following complete immune system destruction increases the duration of long-term remission in patients with multiple sclerosis.

Alvotech prepares for commercial biosimilar production in new facility with single-use bioreactors in Reykjavik, Iceland.

Brammer Bio establishes late-phase development and commercial manufacturing facility for advanced cell and gene therapies in Lexington, MA.

The inactivated vaccine, manufactured with a GE FlexFactory system, could be associated with fewer adverse reactions than the live vaccine option.

Pharmaceutical Technology sat down with Charles N. Kettler, PhD, director of Natoli Scientific, to discuss the Natoli Institute for Industrial Pharmacy at Long Island University.

Customized puresu single-use fluid path assemblies from Watson-Marlow Fluid Technology Group incorporate tubing and BioPure components and are supplied ready to use.

There are technical hurdles to clear in developing serialization and track-and- trace programs, but it’s the human ones that are proving most difficult to surmount.

Effective harvesting and purification processes play an essential role in ensuring that biopharmaceutical manufacturing processes provide biologic drug substances with uniform and consistent properties.

The wearable devices for the delivery of biologic products are now being manufactured and will be tested in clinical trials in the near future, according to the company.

Uncertainty about the demand for a biologic medication can be partly mollified with some well-planned capacity outsourcing, contends a new report by ORC International sponsored by Patheon.
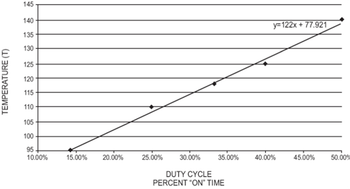
Preheating pinch valves prevents drift in the volume of liquid dispensed.

FDA and bio/pharma companies get serious about continuous manufacturing to ensure product quality.

Industry experts discuss common considerations and recent technological advancements in blow-fill-seal technology.
Process analytical technology paved the way for continuous manufacturing.

Different types of modular systems have advantages and disadvantages.

Industry experts discuss the benefits and challenges of using single-use systems in pharmaceutical manufacturing.

Experts discuss the key considerations in the development of an autoinjector.