
CDER’s Janet Woodcock endorses modern drug manufacturing to ensure access to safe and reliable medicines.

CDER’s Janet Woodcock endorses modern drug manufacturing to ensure access to safe and reliable medicines.

Poly(lactide-glycolide) has been used for drug-delivery applications because of its beneficial physicochemical properties, long safety record, and reliable commercial supply.

Materials and equipment innovations have advanced tablet coating from sugar to copolymers and simplified pharma production.

Real-time alternatives to dissolution testing are required for continuous manufacturing to reach mainstream use.

As commercial manufacturing considers single-use materials, a look shows how industry moved to the technology.

How has the bio/pharmaceutical contract manufacturing industry evolved over the years and what does the future hold?

As pharma models changed during the past 40 years, contract manufacturing capacity and services evolved to meet demand.

The more pharma science and technology change, the more business and policy concerns stay the same.

Biotech-based therapies and a move to single-use processes highlight recent industry changes.

ISPE measures impact of biotechnology and globalization on personalized medicine.

Pharma company consolidation and outsourcing led to a de-emphasis of manufacturing and reduced investment in new technologies and facilities.

Advancements in cell culture and protein technology have opened the door for new therapies.

Pharma’s test of continuous manufacturing is starting with oral solid-dosage forms.

Drug sales forecasts fall for first time in 10 years, thanks to pressures to reduce drug prices and the advent of biosimilars.

Takeda inaugurated expanded capacity of solid-dosage drugs at its site in Oranienburg, Germany.

The European Medicines Agency approved Prezista (darunavir) made via a continuous manufacturing process at Janssen's facility in Puerto Rico.

A survey from The Pistoia Alliance found that just under a quarter (22%) of life-science companies are already using or experimenting with blockchain, but industry collaboration on security and storage standards is needed.

In a new research conducted by SEA Vision and Zenith Technologies, 28% of respondents identified technology selection as the biggest challenge as the industry prepares to meet serialization deadlines.

A loan from the European Investment Bank will give BiondVax resources for Phase III trials and a manufacturing facility for its universal flu vaccine.

The need for flexibility and higher quality are driving advances in parenteral manufacturing and fill/finish equipment.

The company added new products to its InfinityLab LC Series which will be showcased at HPLC 2017 in Prague.

The company has voluntarily recalled Clindamycin Injection USP ADD-Vantage Vials to the hospital/retail level because of a lack of sterility assurance.

A recent meeting emphasized the need for more guidance and flexibility from regulators, as well as harmonization, standards and equipment interoperability.

Aptar now manufactures its child-resistant pump, designed to meet US CPC requirements, at its facility in Congers, New York.
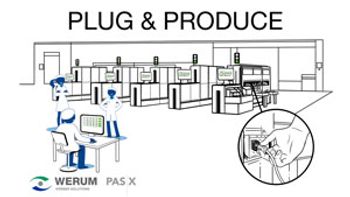
Werum’s Plug & Produce solution eases connection with smart equipment and control systems.