
GE Healthcare continues to ramp up its offerings in the bioprocessing space with the purchase of Asymptote and a continued partnership with Zenith Technologies.

GE Healthcare continues to ramp up its offerings in the bioprocessing space with the purchase of Asymptote and a continued partnership with Zenith Technologies.

Watson-Marlow Fluid Technology Group’s ASEPCO Weirless Radial diaphragm valves eliminate dead legs and product entrapment.
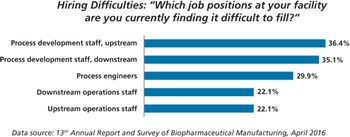
New study shows China biopharma companies face staffing shortages.

The transfer of fluids is governed by different equipment requirements across the medical, biopharma, and cell therapy manufacturing industries.

Renewable energy can improve energy efficiency and reduce carbon dioxide emissions in pharmaceutical manufacturing processes.

The process control and automation requirements of single-use systems differ from those of stainless-steel equipment.

Anil Kane, executive director, Global Head of Formulation Sciences, Pharmaceutical Development Services at Patheon discusses key parameters in the development and manufacturing of oral solid-dosage forms.

The complex nature of biologics adds additional CQAs that must be determined to ensure the safe development of biologics

MDRS differentiates the API from the product matrix and enables measurement of particle size and shape.
As regulators strive for balance in cGMPs for cell, gene, and tissue therapies, risk-management principles must guide decisions involving process media and additives.

Advances in process analytical technology have been achieved, but significant challenges remain.
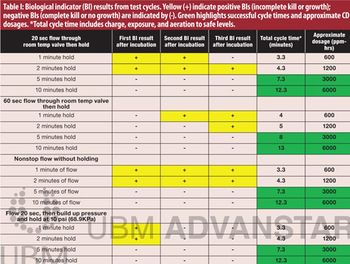
A chlorine dioxide sterilization cycle was developed for a novel split-valve aseptic powder transfer device.

Soft sensors are powerful tools that can be used along with spectroscopic instruments in on-line measurement.

Continuous manufacturing will not work for all pharmaceuticals, but the right infrastructure, senior management support, and planning from the earliest stages of drug development could eventually allow up to 80-90% of small-molecule APIs to be made continuously, says Paul Sharratt, head of process science and modeling at Singapore’s Institute of Chemical and Engineering Sciences.

A venture between GEA and Siemens aims to familiarize more pharmaceutical companies with more modern control and continuous processing.

This novel technology was developed in response to challenges involved in conventional manufacturing of multilayer tablets, including in-line control of the tablet weight, the tendency to delamination, direct contact between the two tablet layers, and cross contamination.

Process analytical technology, based on monitoring particle size distribution and tracking coating thickness measurements in real time, can be used to predict the dissolution of polymer-coated multiparticulates.

The facility in Britley will now manufacture the company’s automated systems, enabling the company to better serve European markets and shorten the supply chain.

Conferences focused on continuous solid-dosage manufacturing aim to spread the word about technical capabilities and alleviate perceptions of regulatory uncertainty.

In a FDAVoice blog post, CBER Director Peter Marks discusses the new designation for cell therapies that treat life-threatening diseases.

Fisher BioServices will expand its CryoHub solution by co-locating it with the Cell and Gene Therapy Catapult manufacturing center for seamless supply chain management and to accelerate cell and gene therapy production.

Richard D. Braatz, PhD, will discuss using mathematical models to design a continuous drug manufacturing plant and the differences between batch and continuous operations for biologics.

Caladrius is selling the remaining percentage of the subsidiary in order to focus on cell therapy development.

The company announced that it would now be offering a portfolio of fresh and cryopreserved human and animal hepatocytes for ADME-Tox testing.

An influx of millennial workers may have an impact on whether pharma manufacturers choose to implement IIoT technology.