
Although best practices are key, advances in integrated informatics platforms and automation can make it easier to ensure data integrity and improve overall lab efficiency.

Although best practices are key, advances in integrated informatics platforms and automation can make it easier to ensure data integrity and improve overall lab efficiency.

The companies have developed a Level 4 traceability solution to manage pharmaceutical regulatory requirements.

SGS expands its elemental impurity testing services at its laboratory in Villeneuve-la-Garenne, France.

Improved process analytical technology and new ways of thinking seek to enhance measurement and control for next-generation pharmaceutical manufacturing.


Modular Automated Sampling Technology (MAST) allows direct aseptic transfer of bioreactor samples to analytical devices, providing rapid and reliable data in bioprocessing.

Although limitations must be overcome, mass spectrometry is having a great impact on biologic development and manufacturing.

Extraction studies demonstrate approaches for evaluating single-use bio-pharmaceutical manufacturing materials.

The authors survey deficiency letters issued for 190 drug master files supporting abbreviated new drug application submissions.
This article introduces the concepts of pooled variance and the central limit theorem, which are intended for establishing acceptance criteria for blend uniformity data of granular powder blends when a significant degree of sampling bias is involved.
The RA802 Pharmaceutical Analyzer from Renishaw is a compact benchtop Raman imaging system that enables users to formulate tablets by speeding up the analysis of tablet composition and structure.

Analytical products for improved bio/pharmaceutical development.

Camfil Air Pollution Control has expanded its testing laboratory and added a dust collection test rig for the ANSI dust collection standard.

B&W Tek’s director of Market and Customer Development, Katherine A. Bakeev, PhD, discusses analysis of pharmaceutical raw materials and the benefits of using handheld Raman spectrometers.

The Uniformity of Dosage Units test is used to evaluate content uniformity or weight variation or dosage forms such as tablets and capsules. The author introduces two different acceptance value limits (n = 10 and 30) in this article.

Agilent’s new facility in Folsom, California includes laboratory, order fulfilment, and warehousing space.

Dedicated facility will address enhanced regulation of metals and impurities in pharmaceuticals.



The CPhI Pharma Award for Excellence in Analysis, Testing, and Quality Control went to B&W Tek for its handheld laser induced breakdown spectroscopy (LIBS) analyzer, NanoLIBS, which has been developed for rapid identification of solid materials based on elemental analysis. The instrument features a raster scan using a high repetition rate class 3B laser allowing for detection of elements including C, Li, and Be.


Visual inspection of parenteral vials is the first step in a root cause investigation.

The Chinese facility was cited for data integrity violations.

New technology introduced in 2016 aids innovation growth for pharmaceutical manufacturing.
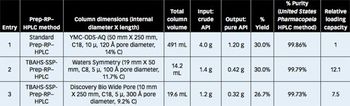
This paper describes a unique Prep-rP-HPLC technique that uses a C-18/C-8 derivatized silica coated with a hydrophobic quaternary ammonium salt or quaternary phosphonium salt that acts as an additional/surrogate stationary phase (AsP/ssP).