
The authors review the solution and powder properties of polyethylene oxide and its various applications in hydrogels and hydrophilic matrix systems.

The authors review the solution and powder properties of polyethylene oxide and its various applications in hydrogels and hydrophilic matrix systems.

New technologies and improvements to existing ones can reduce contamination risk in aseptic processing.

A ready-to-fill closed vial can improve aseptic filling quality and reduce process complexity.
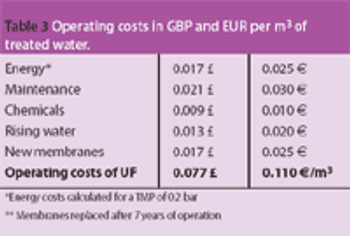
Pure water is a raw material of particular importance to the pharmaceutical industry. Drinking water is the basis for the treatment of water for pharmaceutical applications; it is the starting point for the production of the various pharmaceutical water qualities, such as purified water, highly purified water and water for injection.

The complete elimination of human-derived contamination is possible only with the elimination of human intervention.

Crucell and NIH Sign Ebola Vaccine Manufacturing Contract

The Empire Strikes Back: Innovators Releasing Generic Drugs

HHS Awards $97-Million Vaccine Development Contract

X-ray microtomography has great potential for improving the understanding of the structural features of solid dosage forms and the changes in those features during manufacturing, handling, and storage.This article describes the basic principles of the technique and provides examples of its potential applications.

The sterility testing of samples from an aseptic process may be considered an entirely separate aseptic process that is subject to the same types of adventitious contamination as the aseptic process itself.

An increasing number of new compounds are being introduced into pharmaceutical pilot plants.The knowledge base for these compounds regarding their toxicities,physical handling, and cleaning is limited.The authors examine various approaches for addressing the cleaning validation of new compounds and discuss the role of determining appropriate visible residue limits.

A comparative study of three air samplers used for bioaerosol collection was performed to evaluate the average recovery of colony-forming units and assess the precision of each device.

To meet the requirements of the USP ^755& Minimum Fill and ^698& Deliverable Volume tests, target fill levels greater than 100% must be established.This article proposes a criterion for establishing an appropriate target fill level such that a sample will have a 95% probability of passing these USP tests at 95% confidence.

The authors argue that chlorine dioxide (CD) is a safe and effective decontaminating agent that can be used for challenging applications.The effectiveness of CD gas for sterilizing complex isolator systems is studied.

Innovators and generics makers are staking out positions on follow-on proteins and other manufacturing issues.

Nanotechnology is believed to hold enormous promise for the future of medicine and healthcare...
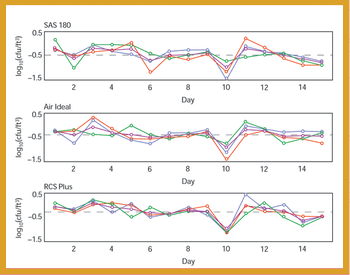
A comparative study of three air samplers used for bioaerosol collection was performed to evaluate the average recovery of colony-forming units and to assess the precision of each device.

Nanostructured lipid carriers are a new type of delivery system offering improved performance in terms of drug loading and long-term stability with the ability to form highly concentrated dispersions...
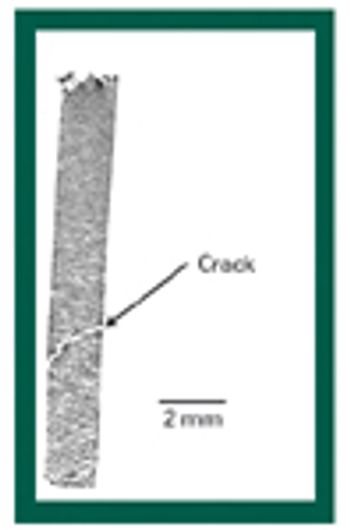
X-ray microtomography has great potential for improving the understanding of the structural features of solid dosage forms and the changes in those features during manufacturing, handling, and storage. This article describes the basic principles of the technique and provides examples of its potential applications.
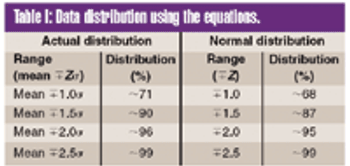
To meet the requirements of the USP ?755? Minimum Fill and ?698? Deliverable Volume tests, target fill levels greater than 100% must be established. This article proposes a criterion for establishing an appropriate target fill level such that a sample will have a 95% probability of passing these USP tests at 95% confidence.

In this article, the author explains some of the technology behind using ion-exchange resins for drug delivery...

This article examines the importance of core design and formulation on the quality of a film coated tablet...

"Stable Liquid" Technology Permits Heptavalent Vaccine Against Botulism

Nanoparticle Synthesis Process Facilitates Chiral Separations

UK MHRA Okays Chiron Flu Vaccine Plant; FDA Approval Still to Come