
FDA Warns Consumers Against Unapproved, Contaminated 'Miracle II Neutralizer'

FDA Warns Consumers Against Unapproved, Contaminated 'Miracle II Neutralizer'
The survival of Bacillus subtilis spores in dicalcium phosphate, lactose, and corn starch and in their binary mixtures depends on the compressional properties of these materials and on parameters involved during the tableting process, including compression speed.

The attraction of nasal administered therapeutic agents is obvious, including faster onset of action, increased compliance and avoiding degradation during first pass metabolism.
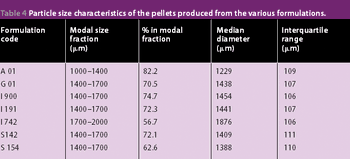
The previous studies on the incorporation of glyceryl monostearate into pellets by extrusion/spheronization has been extended to include a range of grades of this material plus a mixed medium chain partial glyceride and two glycerol esters of hydrogenated natural glycerides as described in this article.

In applying a visually clean standard, any residue related to the cleaning process that is visible on the surface should constitute a failure.
All pharmaceutical products are formulated to specific dosage forms for drugs to be effectively delivered to patients. Typical pharmaceutical dosage forms include oral tablets, capsules, solutions, suspensions, topical ointments, gels, and solutions, and injections for intravenous (IV), intramuscular (IM), or subcutaneous (SC) administration. In addition, various drug delivery systems have been developed for transdermal, intranasal, and pulmonary deliveries. Different dosage forms require different pharmaceutical technologies and usually present different technical challenges for formulation development.
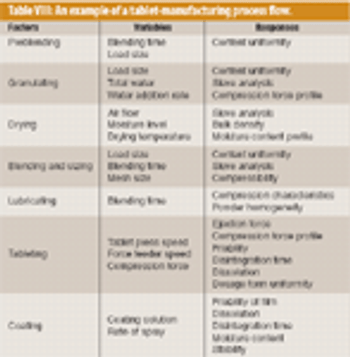
The pharmaceutical industry handles large volumes of granular materials such as powder blends for tablet production and filled capsules everyday (1, 2). Slight changes in ingredient properties or process operation conditions can have a major effect on a finished product's quality. Given the market and regulatory uncertainties that are commonly associated with drug product development, pharmaceutical companies typically have several drugs in various developmental stages at the same time. Because of this volume, the industry must have computer-based rapid-prototyping tools that can efficiently capture and resolve the technical aspects of drug product development so that companies can confidently make decisions about drug portfolio management and planning (3, 4).
Solid oral drug products are one of the oldest of all manufactured dosage forms (1). Today, the development of an appropriate formulation of drug and excipients and of an effective manufacturing process to create a tablet or capsule is slowly transforming from a practice of applied art to one of applied science. The US Food and Drug Administration supports this change by expecting sponsors of new drug applications to understand, describe, and control materials and processes as well as the risks associated with drug product manufacturing (2). These steps will ensure the consistent production of products that meet their specifications and remain safe and effective during their shelf life.

New technology for improved absorption of certain proteins and macromolecules...

Formulators currently face numerous challenges in nanosuspension development in terms of ensuring safety, efficacy, and stability. Presenters at Wednesday's AAPS symposium offered strategies for addressing these challenges, including setting meaningful particle-size specifications, selecting the method to measure particles in nanosuspensions (especially for nonspherical particles), gaining a meaningful particle-size distribution, and determining the particle size from such distributions.

As a pharmaceutical formulation tool, molecular simulation is currently in its early infancy. Nonetheless, presenters at Wednesday?s AAPS Annual Meeting and Exposition demonstrated that the technology is beginning to attract some interest. The topic was discussed in a presentation titled "Application of Molecular Simulations to Formulation Development and Stability Prediction."

After outlining the results of extensive studies on drug-silicate interactions, Robin H. Bogner, PhD, concluded, "We're just scratching the surface." She might have added, "pardon the pun": the effect of silicates' heterogeneous surface chemistry is one of the points of study.

Artium Technologies' (Sunnyvale, CA, www.artium.com) new diode-pumped phase Doppler interferometry systems use solid-state lasers incorporated into transmitting optics, eliminating losses that can result from fiber coupling, alignment, and degradation. According to Atrium, the advantage of this approach to optical design is improved precision and a larger dynamic range, with higher resolution over the entire range.

In the spirit that a good review of the fundamentals is always beneficial, the American Association of Pharmaceutical Scientists' Annual Meeting and Exposition featured an early morning discussion about the basic aspects of dissolution testing, including common sources of errors and deviations. The well-attended session proved that dissolution testing remains a topic of interest, especially as the industry continues to extend its application to media other than solid dosage forms, most notably soft gels.

The International Organization for Standardization (ISO, www.iso.org) has approved the use of the photometric method for liquid delivery performance verification. Artel (Westbrook, ME, www.artel-usa.com), a manufacturer of precision testing and calibration systems for liquid handling instruments, uses the photometric method to calibrate pipettes and automated liquid handlers.

Extending the life cycle of a drug product has paved the way for "the new pharmaceutical industry" according a Tuesday AAPS Annual Meeting session, "Formulation Approaches to Life Cycle Management."

Generally, tablet and capsule film coatings are applied as aqueous or organic-based polymer solutions or dispersions, graduate student Sagarika Bose (University of Connecticut) explained during her Tuesday AAPS Graduate Student Symposium presentation, "Development and Evaluation of Solventless Photocurable Pharmaceutical Film Coating." However, organic film coatings can be flammable, toxic, and must comply with strict environmental regulations. Aqueous film coating can lead to the degradation of certain drugs by heat and water.

At the plenary session at the AAPS Annual Meeting, two researchers presented studies targeting completely different areas of stem cell research, but their work focused on the same ultimate goal: finding new therapies.

When it comes to developing a robust lyophilization process, formulators can "pay now or pay later," says Jeff Schwegman, PhD, founder and chief scientific officer for BioConvergence. Because 30% of new drugs in clinical trials are biotech-based therapeutics (compared with 7% 10 years ago), more than ever, the US Food and Drug Administration is paying close attention to lyophilization data and questioning pharmaceutical companies about their development cycles, especially cycle development transfer, shelf-temperature mapping, dryer-to-dryer comparison studies, formulation time, process validation, and cycle deviation. Consequently, this is pushing formulators to optimize formulation variables, conduct additional testing during early-stage development, and understanding critical process parameters, equipment qualifications, and manufacturing conditions that can influence formulation behavior at a large scale. Not taking the time or effort to achieve these goals during early development could lead to redundancies in formulation work - a reality observed too often in today's practices.

"We must add our light to the sum of lights," declared Ron Reagan in his Nov. 6 keynote address to the 2005 Annual Meeting of the American Association of Pharmaceutical Scientists. He was quoting Billy Kwan, the half-Indonesian, half-Australian photojournalist of divided loyalties in the 1982 film, "The Year of Living Dangerously," a character who redeems himself by taking bold action in the face of moral crisis. Reagan encouraged the audience to take similar action to defend science, which he said is currently subordinated to political convenience.

Palatinit's "galenIQ" is line of multifunctional excipients that offer the combined advantages of other bulk excipients.
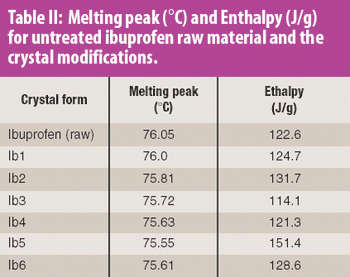
The common crystal form of ibuprofen was changed to optimize processing and manufacturing properties. Six modified crystal forms were prepared and assessed for dissolution, morphology, particle size, density, thermal characteristics, powder x-ray diffractometry, flow properties, and tabletability.

Recent advances in transdermal technologies challenge the paradigm that only a few drugs can be delivered transdermally.
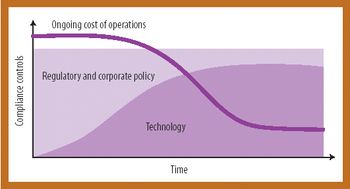
Information technology can streamline compliance and increase operational efficiency and quality.
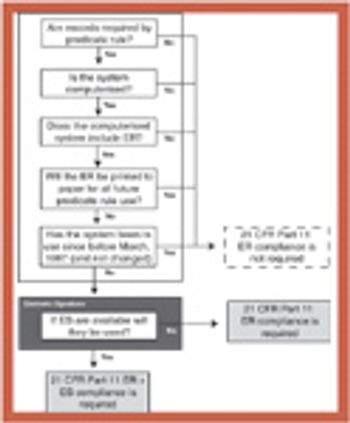
Coordinating validation efforts throughout an organization requires an accurate and timely overview and a validation master plan (VMP).