
Purdue?s RFID Pedigree Program Enters Pilot Phase

Purdue?s RFID Pedigree Program Enters Pilot Phase

Wet granulation is a size-enlargement process in which a liquid is used to achieve the agglomeration of solid particles. Agglomeration improves particles' tableting properties by rendering them free-flowing, nonsegregating, and suitable for compression (1).
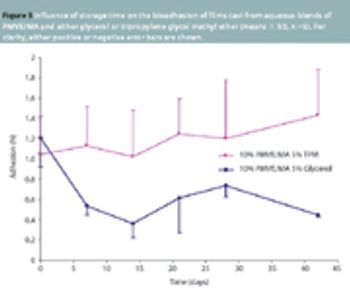
Bioadhesive films cast from aqueous blends of PMVE/MA have diverse uses, such as a means of establishing an electrically conducting interface for bioelectrodes and as an adhesive drug delivery matrix.
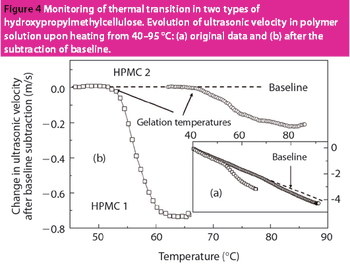
HR-US is a nondestructive technique with enormous potential for the analysis of materials and formulations used in the pharmaceutical industry.
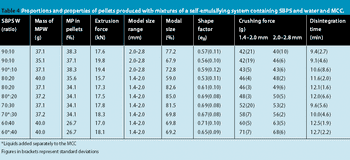
This high quality of pellet roundness is surprising in view of the high extrusion forces required to extrude the wet masses.

Glossary of computer and software terms.

New Excipient Guidance Doesn?t Fill Regulatory Gap

Recalls, Resignations, and Suspended Manufacturing Plague Able Labs

Compounding Pharmacies' Lawsuit Against FDA Will Continue

FDA Drug Safety Oversight Board

It Helps to be Flexible: Supple WIN Compounds Could Cure the Common Cold

Cytogen and Dowpharm to Develop Targeted Anticancer Product


Bedford Recall

GSK and FDA Agree on Consent Decree
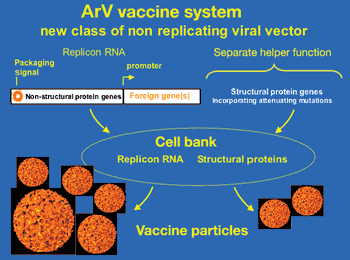
Vaccine developers are using novel drug delivery methods that offer advantages over traditional techniques such as improved immunogenicity, better stability, specific control over antigen release, and a wider pool of targeted diseases.

Vaccine developers are wrking on new drug delivery systems that offer improved immune responses, better stability, and a wider pool of targeted diseases.

The authors review the solution and powder properties of polyethylene oxide and its various applications in hydrogels and hydrophilic matrix systems.

The unit-dose bar coding rule requires integrating many aspects of packaging design and control.

FDA is finalizing guidances and enforcing compliance with manufacturing standards as part of its efforts to ensure drug safety.

A newly developed software program transforms written SOPs for all required analytical method validation experiments into transferable automated templates, integrating individual activities and technologies under one platform.

Major pharmaceutical companies are focusing more than ever on the prices CROs are charging for their services.

New technologies and improvements to existing ones can reduce contamination risk in aseptic processing.

A ready-to-fill closed vial can improve aseptic filling quality and reduce process complexity.

The complete elimination of human-derived contamination is possible only with the elimination of human intervention.