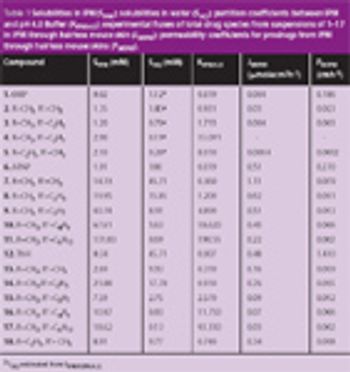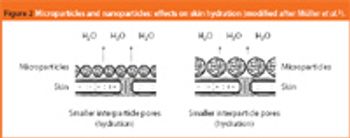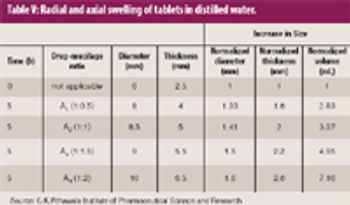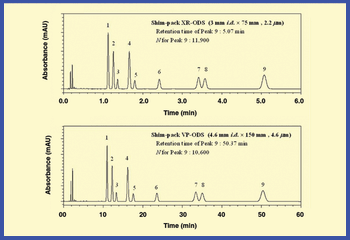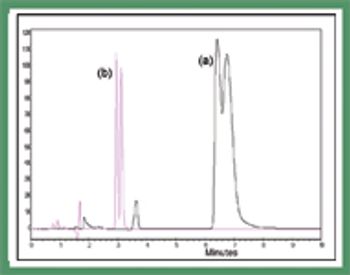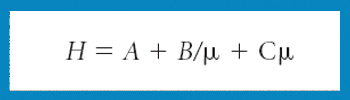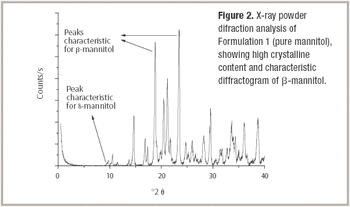
Mannitol is the most commonly used bulking agent in freeze-drying formulation design. The benefit of using mannitol is that it crystallizes during freezing and permits drying processes at higher product temperatures, and thus with higher sublimation rates relative to purely amorphous systems (1). Mannitol, however, is known to form different crystalline modifications which compromises reproducibility of product characteristics and storage stability due to phase transformations (2, 3).