
Researchers develop various catalytic approaches for improving yield, purity, stereoselectivity, and process conditions.

Researchers develop various catalytic approaches for improving yield, purity, stereoselectivity, and process conditions.
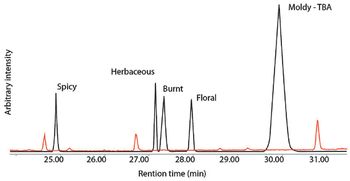
Combining olfactometry analysis with multidimensional gas chromatography–mass spectrometry provides an extremely useful analytical method for identifying aroma or odor notes from a sample.

A Technical Forum Moderated by Patricia Van Arnum, featuring contributions from PerkinElmer, BioTools, Chiral Technologies, Shimadzu Scientific Instruments, GE Analytical Instruments, and Waters.
The authors investigate the effect of low pH and ionic strength on aggregation using turbidity measurements and size-exclusion–high-performance liquid chromatography.

Could oral absorption-enhancing technologies change the shape of protein delivery?

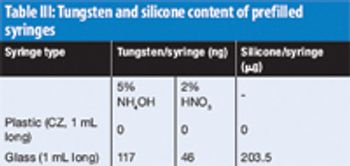
New product reviews for October 2011 focusing on analytical instrumentation.

The global excipients market shows moderate growth, increased consolidation, and expansion activity in emerging markets and select product areas.

Where are the new excipients, the new solubilisers and sustained release excipients?

Biocatalysis, chemocatalysis, and other chiral technologies continue to attract the investment dollars of CMOs and fine-chemical companies.

Direct dosing APIs during R&D studies can reduce the overall testing time of a drug candidate by allowing for a greater throughput of compounds through the R&D department.

The increasing cost of crucial manufacturing input factors, such as energy and raw materials, has been a severe threat to several companies.

Excipients are the hidden champions of drug development-no API works consistently without the right excipient. Pharmaceutical excipients, however, require stringent quality management. This article discusses how the supplier of pharmaceutical raw materials should take a central role in ensuring excipient quality.

As contract manufacturers and fine-chemical suppliers gather for CPhI/ICSE, effective strategies for technology differentiation are key in an increasingly competitive environment.
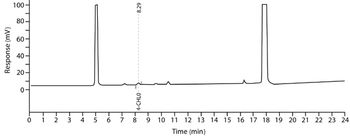
The authors provide an overview of methods for the quantitative determination of genotoxic impurities (GTIs) in active pharmaceutical ingredients.

Recently, there have been many innovations in the latest techniques and technologies in API purification. In particular, the trend to single-use systems has had a significant impact on processes.
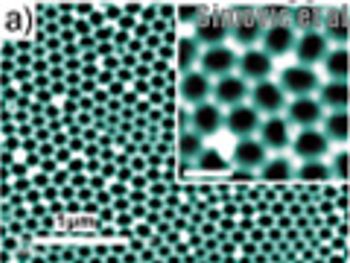
The authors present a method for controlling the release of therapeutics by applying a plasma polymer layer to the surface of porous materials.

O-arylation and O-alkylation, a one-pot protein synthesis, a combined approach in continued and chemocatalysis, and green-chemistry applications are the target of some recent advances in API synthesis.

The solid form of an API plays a crucial role in drug quality, and advancing methods for screening, detection, and characterization is key.

Two popular methods for detecting protein aggregates are analytical ultracentrifugation (AUC) and size-exclusion chromatography?multiangle light scattering (SEC?MALS). These techniques? results correlate relatively well, but each one has its own strengths.

Listen to roundtables from the 2011 ExcipientFest/IPEC conference, addressing developing issues in excipient functionality and continuous manufacturing.
More sophisticated biological expression systems expand the functionality of the traditional systems for protein synthesis.

Effective containment in API and drug-product manufacturing encompasses a variety of process, equipment, and operational issues.

The IPEC is soliciting public comment about a draft plan for the independent certification of manufacturers and suppliers of pharmaceutical excipients.
The authors examine the influence of glass-transition temperature, melt viscosity, degradation temperature, and process settings.

Approaches in using small-molecule and peptide synthesis offer promise in widening the scope of drug candidates.