
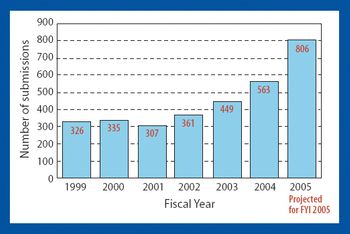
CDER Creates Office of New Drug Quality Assessment

CDER Creates Office of New Drug Quality Assessment

Ajaz Hussain Leaving FDA for Sandoz
The US Food and Drug Administration (Rockville, MD, www.fda.gov) released a new draft guidance that may speed generic approvals. The guidance, ANDAs: Impurities in Drug Products, describes the degradation-product information that generic drug manufacturers should include in their abbreviated new drug applications (ANDAs). This clarification, FDA officials say, will help companies submit the correct information, thus increasing the likelihood that their generic drugs will be approved, and approved more quickly.

One of the world leaders in the manufacture of tablet compression tooling, I Holland, is offering a range of machines for the automatic polishing of tablet compression tooling.
Adding Braille to pharmaceutical packaging should be less of a challenge with the use of Esko-Graphics' Scope solution. EC Directive 2004/27/CE requires Braille labelling and information to be provided with pharmaceutical products for human use, and companies are scrambling to implement this by 31 October.

Biogen to Cut Jobs

The US National Institute of Allergy and Infectious Diseases (NIAID, Bethesda, MD, www3.niaid.nih.gov) announced it will order two million doses of an avian influenza vaccine from Sanofi-Pasteur (Swiftwater, PA and Lyon, France, www. sanofipasteur.com). In April, the NIAID began a Phase I trial to evaluate the vaccine’s safety and ability to generate immunity against the H5N1 strain of avian flu, an illness that leads to severe disease and possible death in birds and humans.

Manufacturers of pharmaceuticals and nutraceuticals can protect their products from moisture and other elements while also enhancing marketability, by adding scent to their packaging. The full line of scented packaging solutions, offered by Süd-chemie, is reported to be ideal for applications that emit an unappealing odour to end users. The packaging can also enhance the marketability of odourless products. The flagship product in the scented solution line is Aroma-Can — a product modelled after the company's desiccant canisters. One-gram canisters will be available in orange and lemon scented variations, and can be inserted at the same high rates of speed as traditional desiccant canisters using standard canister insertion equipment.

Two of the largest companies in the pharma industry are to join forces to supply and distribute 96-well Multi-SPE Extraction Plates. The instruments, which are to be circulated by Millipore, are manufactured by 3M for the purpose of isolating and concentrating low levels of analytes, and function by removing substances that interfere with mass spectrometry signal detection. The devices feature 3M Empore membranes, which produce clean extracts, extend LC-MS/MS column life and decrease instrument downtime.

Chiron Corporation (Emeryville, CA, www.chiron.com) announced that quality problems at its Marlburg, Germany, manufacturing plant will prevent the company from supplying its "Begrivac" influenza virus vaccine to non-US markets for the 2005–2006 flu season.

To help meet the needs of the fast-growing, global biopharmaceuticals industry, Pall Corporation has launched five innovative technologies aimed at increasing drug-manufacturing efficiency through customization and disposability. The Mustang XT5000 capsule is the company's novel chromatography technology for efficient capture of large molecules, especially used to develop DNA and virus-based drugs. Expanding its portfolio of technologies for disposable processing, Pall offers both the customizable Tangential Flow Filtration system, which incorporates single-use components for downstream processing applications, and the Kleenpak Connector — a new single-use device, which enables aseptic connections to be made instantly.

Colorcon has opened three specially equipped design centres devoted to tablet brand imaging. Located in West Point (PA, USA), Dartford (UK) and Goa (India), the facilities form an integral part of the brand enhancement system for tablets — a unique service supporting new product development and brand management of an existing product. Each design facility is equipped for collaboration between Colorcon experts and a client's own project team.

Aptuit To Purchase Quintiles

Able Labs Files for Chapter 11

Xanodyne Acquires aaiPharma Assets


Able Interim Chief Resigns Amid Drug Recall

Able Laboratories, Inc. (Cranbury, NJ, www.ablelabs.com) announced in late May that it would halt all production and recall all available product because of problematic testing procedures discovered during an internal investigation. The company manufactures mostly generic prescription drugs, including drugs containing acetaminophen.

Schering AG To Cut Plants and Staff

Wyeth and Purdue Announce Restructuring Plans

Pharmaceutical science and technology news.

Regulation: FDA Steps Up Action Against Counterfeit Drugs, Warns US Consumers Against Fake Medicine from Mexico

Once considered mainly an afterthought in a company's lifecycle-management strategy, controlled-release dosage forms are now positioned at the forefront of many formulation strategies. In contrast to drug discovery, formulation work focuses not only on the intricacies of the active pharmaceutical ingredient (API), but also on fine-tuning the excipients, the release profile, and the delivery mechanism to provide optimal therapeutic benefit. Because of their wide range of applications and functionalities, especially in controlled-release therapies, polymers are among the most widely used excipients.

Purdue?s RFID Pedigree Program Enters Pilot Phase

Compounding Pharmacies' Lawsuit Against FDA Will Continue

Cytogen and Dowpharm to Develop Targeted Anticancer Product

Bedford Recall

GSK and FDA Agree on Consent Decree

Pharmaceutical science and technology news.

A ready-to-fill closed vial can improve aseptic filling quality and reduce process complexity.