Pharmaceutical Technology Editors
Articles by Pharmaceutical Technology Editors

Recent EU legislation change has opened the door for UK firm Accentus to use its novel predictive crystallization technology to discover and develop generic alternatives to patented drugs. Accentus will also licence its CrystalGEM predictive crystallization technology to generics manufacturers.

Pharmaceutical industry regulation is about to change with a new code of practice. This has been revealed by the Association of the British Pharmaceutical Industry (ABPI) following recent problems with patient safety and the dissemination of data and information of approved drugs, which exposed loopholes within the code.
Merck (Whitehouse Station, NJ, www.merck.com) has revealed the "first phase" of its global restructuring program set to eliminate 7000 jobs (11% of its global workforce) by the end of 2008, close or sell 5 of its 31 manufacturing facilities, and roll out a manufacturing strategy to "drive significant efficiencies, decrease headcount, and reduce or refocus operations throughout the plant network and the entire manufacturing division." The company also expects to close one basic research site and two preclinical development sites.

Amgen Losing Hold on Anemia Drug Market

Bristol-Myers Squibb to Cut Costs by $500 Million

Roche Selects 12 Potential Manufacturing Partners for Tamiflu

SEC Loosens Revenue-Recognition Rules for Vaccine Stockpile Participants

NIAID Boosts Vaccine Innovation but Draws Controversy

FDA Warns Consumers Against Unapproved, Contaminated 'Miracle II Neutralizer'

From politics to paychecks and downsizings to deadlines-what it's like to work for one of the world's largest industries.

Roche (Basel, Switzerland, www.roche.com) has put a temporary halt on the distribution of the antiviral treatment, "Tamiflu" (oseltamivir phosphate), a neuraminidase inhibitor, to the United States in an effort to deter companies from stockpiling the antiviral for employee use, according to an article in the Oct. 27 edition of The New York Times.

With the prospect of a possible pandemic the Swiss company, Roche, has found sales of its flu drug have rapidly increased but its patent is being questioned. Roche's Tamiflu sales have inflated by 263% in the first 9 months of this year but discussions of patent suspension from several countries and United Nations Secretary General Kofi Annan is pressurizing the company.

The introduction of new measures on advertising and promotional materials for prescription medicines could delay UK launches. The Medicines and Healthcare products Regulatory Agency (MHRA) has admitted that the checks, which have been introduced in response to a Commons' Health Select Committee report, could extend a launch by at least 2 weeks.

The monopoly held by the large pharmaceutical companies within drug discovery could be falling into the hands of biotechnology firms claims Frost and Sullivan. The consultancy company's report, Biochips Technology Redefines Process of Drug Discovery, states that biochip manufacturers are encouraging end users to accept the new technology by providing novel and effective solutions.

OTC Eye-Drop Maker Signs Consent Decree

Merck to Close Plants, Slash Jobs, Streamline Manufacturing

American Pharmaceutical Partners to Merge with American BioScience

GSK Study Questions Bioequivalency of Generic Cold Sore Creams

Pfizer to Shut Parsippany Plant

Adjuvant for HPV Vaccine Enhances Immune Response Levels

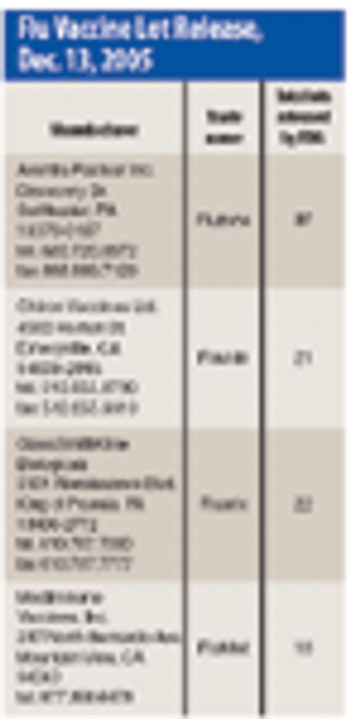
Chiron Looks to Flu Vaccine Cell Culture; FDA Gears Up

Continuous manufacturing processes?little used in the pharmaceutical industry but the norm in oil, food, chemical, and polymer manufacturing?go hand-in-hand with the current emphasis on quality-by-design and automated process monitoring and control (aka, process analytical technology, PAT).

The process analytical technology (PAT) initiative has been percolating at the US Food and Drug Administration for a long time, explained FDA's John E. Simmons at the AAPS Annual Meeting and Exposition on Wednesday. "If you think of PAT as an isolated set of applications, I think you are missing the point," Simmons said. "The FDA would like PAT to become commonplace?not to be an initiative, but common practice."

This week, the US Food and Drug Administration posted an Oct. 20 Warning Letter (http://www.fda.gov/foi/warning_letters/g5567d.htm) sent by its Minneapolis, MN district office to Diversified Manufacturing Corporation (Newport, MN).
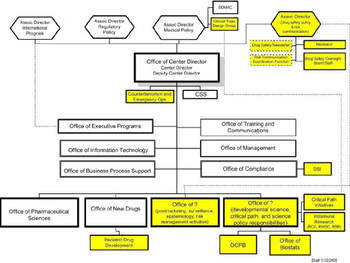
CDER 2005 Product Approval Totals Proposed CDER Reorganization

Formulators currently face numerous challenges in nanosuspension development in terms of ensuring safety, efficacy, and stability. Presenters at Wednesday's AAPS symposium offered strategies for addressing these challenges, including setting meaningful particle-size specifications, selecting the method to measure particles in nanosuspensions (especially for nonspherical particles), gaining a meaningful particle-size distribution, and determining the particle size from such distributions.

As a pharmaceutical formulation tool, molecular simulation is currently in its early infancy. Nonetheless, presenters at Wednesday?s AAPS Annual Meeting and Exposition demonstrated that the technology is beginning to attract some interest. The topic was discussed in a presentation titled "Application of Molecular Simulations to Formulation Development and Stability Prediction."

Control Development's (South Bend, IN, www.controldevelopment.com) blend uniformity and dryer monitor consists of a NIR spectrometer, a sampling head, computer, and software.

After outlining the results of extensive studies on drug-silicate interactions, Robin H. Bogner, PhD, concluded, "We're just scratching the surface." She might have added, "pardon the pun": the effect of silicates' heterogeneous surface chemistry is one of the points of study.

Artium Technologies' (Sunnyvale, CA, www.artium.com) new diode-pumped phase Doppler interferometry systems use solid-state lasers incorporated into transmitting optics, eliminating losses that can result from fiber coupling, alignment, and degradation. According to Atrium, the advantage of this approach to optical design is improved precision and a larger dynamic range, with higher resolution over the entire range.







