
Gottlieb challenges industry. First GenerationNext Awards. Implementing PAT. Optimizing site risk. RFID in the supply chain. Biodegradable polyketals. Global drug-market growth moderates.

Gottlieb challenges industry. First GenerationNext Awards. Implementing PAT. Optimizing site risk. RFID in the supply chain. Biodegradable polyketals. Global drug-market growth moderates.

Bang & Olufsen Medicom (Copenhagen, Denmark, www.medicom.bang-olufsen.com), a drug-delivery device solutions provider, and Bespak (Milton Keynes, UK, www.bespak.com), a medical-devices and inhalation-valve technologies company, established an exclusive partnership to codevelop and comarket the ?Assist Actuated Inhaler,? a single-increment, dose-counting inhaler. The integrated device features an assisted firing mechanism for easier patient use, according to a company release.

Agilent Technologies Inc. (Palo Alto, CA, www.agilent.com) announced April 17 that it acquired SynPro Corp. (Boulder, CO), a contract manufacturer of oligonucleotide active pharmaceutical ingredients. Earlier, Dalton Pharma Services (Toronto), announced that it had completed a multi-gram CGMP oligonucleotide production facility.

Millipore Corporation (Billerica, MA, www.millipore.com) and Serologicals Corporation (Norcross, GA, www.serologicals.com) have announced an agreement in which Millipore will acquire Serologicals in a cash transaction valued at approximately $1.4 billion and set to close by June 30, 2006.

Cardinal Health, Hospira, Siemens, Ranbaxy, World Health Organization

On April 24, Antares Pharma (Exton, PA, www.antarespharma.com) unveiled its new ?TecTix? advanced transdermal delivery system for the transport of topical drug and excipients across the skin by changing the melting point of the active ingredient. Whereas previous methods focused on the delivery of the drug through the dermis and directly into the bloodstream, the TecTix method can be used locally for dermatological treatments and local anesthetics.

Abraxis BioScience (Schaumburg, IL, www.abraxisbio.com) has entered into a definitive agreement to purchase Pfizer?s (New York, NY, www.pfizer.com) Cruce D?vila manufacturing facility in Barceloneta, Puerto Rico.

The US Food and Drug Administration (Rockville, MD, www.fda.gov) will hold a public meeting in the fall of this year to gather information about current developments in the uses of nanotechnology materials in FDA-regulated products.

Interphex Mexico, Pfizer, Dalton Pharma Services...

Boca Medical Products, Inc. (Coral Springs, FL) has initiated a recall of 3984 boxes of its 30-g, 0.5-cc "Ultilet" insulin syringe.

Pfizer, Stevens Institute of Technology, Sanofi-Aventis...

The US Food and Drug Administration's Center for Biologics Evaluation and Research (CBER) is again extending an invitation to biologics facilities for participation in its Regulatory Site Visit Training Program (RSVP). The program, initiated in 2005, aims to give CBER's regulatory review, compliance, and other relevant staff an opportunity to visit biologics facilities and allow CBER staff to directly observe routine manufacturing practices. The objective is to give its staff a better understanding of the biologics industry, including its challenges and operations, and improve communication with CBER staff and industry.

On Feb. 21, the US Food and Drug Administration issued a warning letter to Wockhardt (Mumbai, India), a drug manufacturer. FDA cited the company for violations of CGMPs in the manufacture of drug products and active pharmaceutical ingredients.

The Chemical Heritage Foundation and the Biotechnology Industry Organization presented the 8th Annual Biotechnology Heritage Award to Alejandro Zaffaroni, PhD, a biotechnology pioneer and entrepreneur. The award was presented on April 10 at BIO 2006 in Chicago.

Reverse Genetics Avian Influenza Vaccine Approved in France

GSK Begins Clinical Trials of Two H5N1 Vaccines

Company Notes for Apr. 6, 2006

Eisai plans $105-million plant. Novartis to build plant in China. BMS building $660-million biologics facility. Slow adoption of RFID. Vaccines update. $14.7 billion in pharma construction in 2006. Chiron sells Betaseron to Schering. Baxter to develop cell based H5N1 vaccine. Drug sales up 5.4%, but growth slowing.

New Biodegradable Polyketals Developed for Drug Delivery

Progress Continues in Vaccine and Antiviral Development

First GenerationNext Awards at Interphex 2006

Spotlight on Products from Interphex

Implementing a PAT Strategy

RFID and the Future of Pharmaceutical Supply Chains

Gottlieb Challenges Manufacturers to Make Processes More Efficient

Interphex FasterCures Keynote Speaker Stresses Communication and Collaboration

Development Begins on Mutated H5N1 Virus Vaccine

Proposed Legislation to Help Fight Counterfeit Drugs
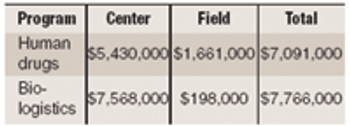
Flu vaccines. Dow veterinary vaccine made in plants. $1.95 billion FDA budget focuses on high-profile programs. Non-injectable insulins win approval. FDA releases guides to dissolution testing, quality overall summary, and new drug-label formats.
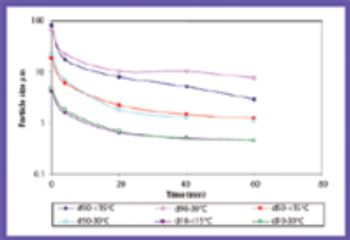
A modified modular high-pressure system can form nanosuspensions of model compounds by individually controlling cavitation, impact, and shear forces.