Pharmaceutical Technology Editors
Articles by Pharmaceutical Technology Editors

In the spirit that a good review of the fundamentals is always beneficial, the American Association of Pharmaceutical Scientists' Annual Meeting and Exposition featured an early morning discussion about the basic aspects of dissolution testing, including common sources of errors and deviations. The well-attended session proved that dissolution testing remains a topic of interest, especially as the industry continues to extend its application to media other than solid dosage forms, most notably soft gels.

The International Organization for Standardization (ISO, www.iso.org) has approved the use of the photometric method for liquid delivery performance verification. Artel (Westbrook, ME, www.artel-usa.com), a manufacturer of precision testing and calibration systems for liquid handling instruments, uses the photometric method to calibrate pipettes and automated liquid handlers.

Extending the life cycle of a drug product has paved the way for "the new pharmaceutical industry" according a Tuesday AAPS Annual Meeting session, "Formulation Approaches to Life Cycle Management."

Generally, tablet and capsule film coatings are applied as aqueous or organic-based polymer solutions or dispersions, graduate student Sagarika Bose (University of Connecticut) explained during her Tuesday AAPS Graduate Student Symposium presentation, "Development and Evaluation of Solventless Photocurable Pharmaceutical Film Coating." However, organic film coatings can be flammable, toxic, and must comply with strict environmental regulations. Aqueous film coating can lead to the degradation of certain drugs by heat and water.
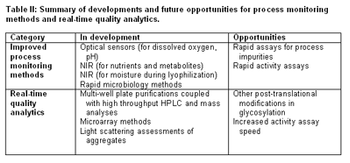
"The better we understand the relationship between process parameters and product attributes, the better control we'll have over product quality," said Beth Fowler, PhD, during Tuesday?s session on process monitoring at the AAPS Annual Meeting.

At the plenary session at the AAPS Annual Meeting, two researchers presented studies targeting completely different areas of stem cell research, but their work focused on the same ultimate goal: finding new therapies.

Pressures to save API are driving formulation developers toward smaller-scale laboratory processes, while pressures to save time put a premium on more- accurate laboratory scale tools.

"In my experience, you can generally tell where a person stands on the issue by the example he gives," said Art Mlodozeniec, PhD, a panelist at the Nov. 7 roundtable on follow-on biologics at the AAPS Annual Meeting in Nashville, Tennessee. "If he brings up human growth hormone and says the processes and impurities are easy to control, he's from the generic industry and supports approval for follow-on biologics. If he brings up the challenge of of erythropoeitin, he's from the innovator industry and opposes generics."
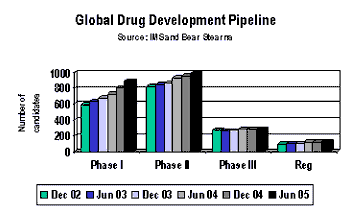
Contract services providers gained a valuable look into major market conditions, offshoring trends, and 2006 growth opportunities during a morning presentation entitled "CMC Sourcing in transition: Consolidation, Offshoring, and the Market Outlook," presented by Jim Miller. Miller is president of PharmSource (Springfield, VA, www.pharmsource.com), publisher of the Bio/Pharmaceutical Outsourcing , and contributing editor of Pharmaceutical Technology.

Thermo Electron (Waltham, MA, www.thermo.com) debuted the "LCQUAN 2.5" data-acquisition system at the AAPS Annual Meeting and Exposition on Monday. The new system expands the software offerings for the company's "Finnigan TSQ Quantum" series of triple quadrupole mass spectrometers.

"We must add our light to the sum of lights," declared Ron Reagan in his Nov. 6 keynote address to the 2005 Annual Meeting of the American Association of Pharmaceutical Scientists. He was quoting Billy Kwan, the half-Indonesian, half-Australian photojournalist of divided loyalties in the 1982 film, "The Year of Living Dangerously," a character who redeems himself by taking bold action in the face of moral crisis. Reagan encouraged the audience to take similar action to defend science, which he said is currently subordinated to political convenience.

Palatinit's "galenIQ" is line of multifunctional excipients that offer the combined advantages of other bulk excipients.

Roche Puts Hold on Tamiflu in US

11 Universities Join FDA ?National Institute for Pharmaceutical Technology and Education?

US District Court Rejects FDA Suit to Block Device Manufacturer

Novartis Purchases Chiron in a Total Buyout

Moheb Nasr, PhD, director of the newly created Office of New Drug Quality Assessment at the US Food and Drug Administration (Rockville, MD, www.fda.gov), has proposed creating a "regulatory agreement" between FDA and sponsors to govern the chemistry, manufacturing, and controls (CMC) sections of new drug applications (NDAs).
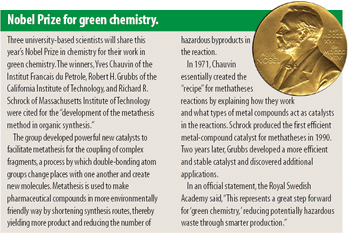
Making active pharmaceutical ingredients (APIs) requires long chains of chemical reactions and large quantities of solvents. Ask API manufacturers how they'd like to improve this process, and the responses are likely to be "make the reactions faster," "make the reactions cheaper," or "make the reactions more efficient." Then after all these economically driven answers, you might here, "make the reactions more environmentally friendly."
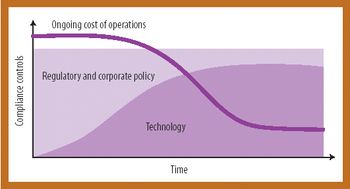
Information technology can streamline compliance and increase operational efficiency and quality.

A range of insect exterminator equipment has been developed to improve the level of hygiene offered to the pharmaceutical and chemical manufacturing sectors. Berson's Insectron range uses ultraviolet (UV-A) and green light to attract flying insects, which are sensitive to these light sources.

Sigma-Aldrich Fine Chemicals (SAFC) has set up a new business division called SAFC Biosciences to encompass its biopharmaceutical development and cell culture-related manufacturing services.

Lester Crawford, FDA's top man, has resigned explaining that it was time to step down — despite only being promoted to commissioner earlier this year.

FDA's Evans Reviews Causes of Warnings and Recalls

Roche Plans New US Manufacturing Facility for Tamiflu while Cipla Prepares to Market Generic

Patheon, CEPH Begin Corrective Action Program for Omnicef OP

New Legislation To Ensure Stable Flu Supply

USP Announces Monograph Revisions List and Postponed Publication of General Chapter <1226>

FDA Proposes New CMC ?Regulatory Agreement?

Design Exhibition, ?From Master?s Thesis to Medicine Cabinet?

CEPH International Replies to FDA Warning Letter










