
The authors present the results of a survey of small- and large-molecule pharmaceutical and biopharmaceutical companies on implementation of Analytical quality by design concepts.

The authors present the results of a survey of small- and large-molecule pharmaceutical and biopharmaceutical companies on implementation of Analytical quality by design concepts.
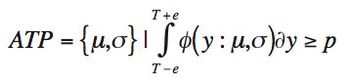
The quality-by-design principles used to control process variability are equally important to measurement systems because process variability includes contributions from measurement system variability. The authors use real-life examples from drug development projects to outline how an understanding of chromatographic measurement system variability might be achieved.
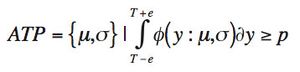
Published: September 2nd 2014 | Updated:

Published: April 2nd 2017 | Updated: