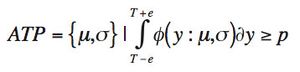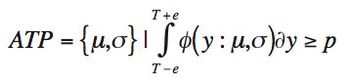
The quality-by-design principles used to control process variability are equally important to measurement systems because process variability includes contributions from measurement system variability. The authors use real-life examples from drug development projects to outline how an understanding of chromatographic measurement system variability might be achieved.