
Tools help improve understanding of excipient risk in formulating OSD drugs.

Tools help improve understanding of excipient risk in formulating OSD drugs.
Understanding European GMPs and new rules from China for excipients are crucial for formulating solid-dosage drugs.

The bill includes multiple less-noticed provisions to bolster healthcare programs and to advance the development of new treatments and preventives to combat the virus.

AstraZeneca has announced that it has achieved approval in Japan for Lokelma (sodium zirconium cyclosilicate) for the treatment of patients with hyperkalemia (elevated blood potassium levels).

Noting traditional clinical trials for COVID-19 convalescent plasma will take time, FDA is allowing physicians to submit requests for single-patient emergency INDs.

Bayer, Novartis, Mylan, Teva, report that they are working on supply.

In light of the current COVID-19 pandemic, the agencies co-chaired the first global regulators meeting to facilitate development of vaccines against SARS-CoV-2, which causes COVID-19.

FDA officials are rolling out guidance and support for researchers striving to assess potential treatments for COVID-19 while the agency tries to object to premature optimism and regain public credibility.

FDA has been working closely with other government agencies and academic centers that are investigating the use of the drug chloroquine to determine whether it can be used to treat patients with mild-to-moderate COVID-19.

FDA is offering advice and added flexibility to help sponsors adjust ongoing and planned clinical research programs during the COVID-19 outbreak.

In a daily press briefing on March 18, 2020, the World Health Organization (WHO) announced that, along with its partners, it will be organizing a large international study that will compare various untested treatments for COVID-19.

Synairgen, a respiratory drug discovery and development company, has announced that it has received expedited approvals to conduct a trial of its inhaled formulation of interferon-beta-1a, SNG001, in COVID-19 patients.

The guidance details considerations FDA has set in place to assure the safety of participants while maintaining compliance with good clinical practices and minimizing risks to trial integrity.

The Pharmacovigilance Risk Assessment Committee (PRAC) of EMA has recommended that women stop taking 5-mg ulipristal acetate (Esmya and generic medicines) for the treatment of uterine fibroids while a safety review into potential liver injury risk is performed.

Following reports, that are particularly circulating social media, questioning whether non-steroidal anti-inflammatories (NSAIDs), such as ibuprofen, worsen COVID-19, the European Medicines Agency (EMA) has issued advice.

FDA postpones routine domestic facility inspections due to the COVID-19 pandemic.

The test will use Applied Biosystems TaqPath Assay technology and will work to provide patient results within four hours of a sample being received by a lab.
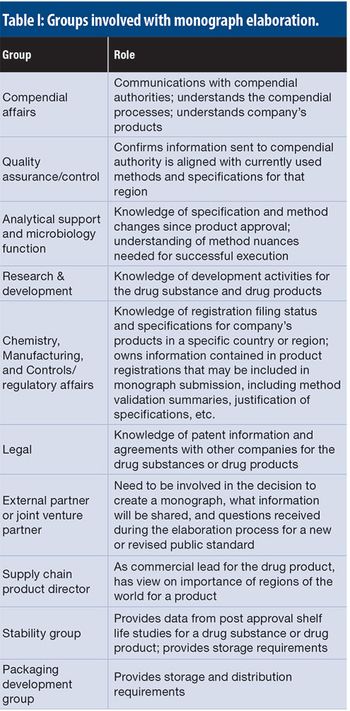
This article details the more operational aspects of monograph submissions, answering the question of how to participate.

The authors present a case study with raw materials and excipients, where a consistent, cross-functional approach is needed to ensure the appropriate selection, sourcing, testing, and filing of the materials used to manufacture bio/pharmaceutical products in a global environment, ensuring compliance with applicable compendial and regulatory requirements.

This article summarizes all the considerations that go into a company’s compendial affairs program and to look ahead at topics that will likely result in further evolution in the pharmacopoeias around the world. This look into what is on the horizon is important to help companies prepare for the inevitable changes and ensure the continued supply of quality medicines to patients globally.

This series is intended to address the challenges for the industry to comply with pharmacopoeial requirements. This article returns to this important topic with a case study at the intersection of monograph development and compliance.

Find links to pertinent regulatory and standard setting resources, guidance documents, and guidelines.
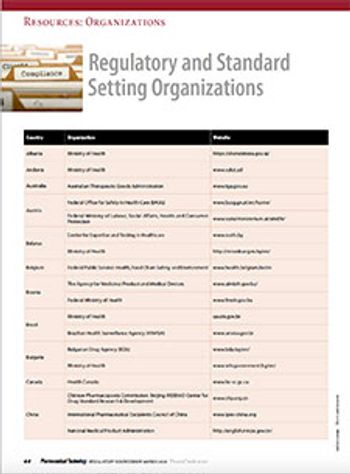
Connect with pharmaceutical and healthcare regulatory authorities around the world via this directory.

With some FDA inspections on hold, will the US drug supply maintain its quality standards?

Monographs are developed based on the submission of information and materials from a company having regulatory approval for the product, and this submission feeds into the pharmacopoeia revision process.