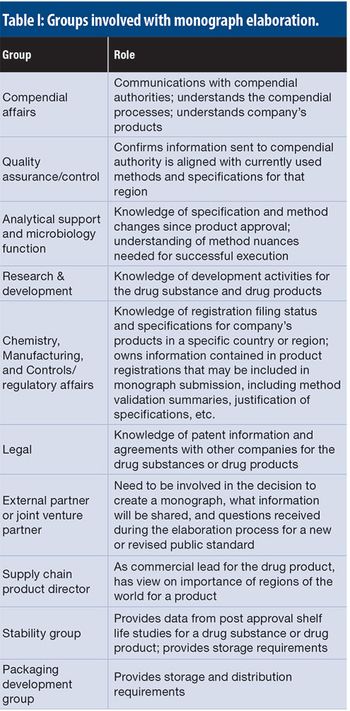
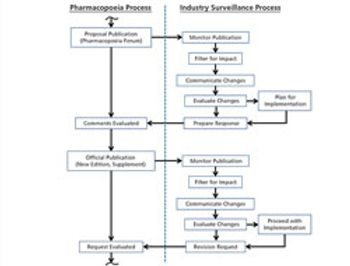
This paper provides a technical assessment of compendial tests commonly found in the European Pharmacopoeia, the United States Pharmacopeia–National Formulary, and the Japanese Pharmacopoeia, detailing differences between the methods and acceptance criteria, and the potential impact of these differences on multi-compendial compliance.