
FDA testing has found an additional impurity, N-Nitrosodiethylamine, in the API valsartan.

FDA testing has found an additional impurity, N-Nitrosodiethylamine, in the API valsartan.

FDA is revising its inspection process and seeks harmonization of standards for US and foreign regulatory oversight to ensure the safety of medicines.

FDA Commissioner Scott Gottlieb, MD, issued a statement regarding new warning letters FDA issued to Chillin Mix Kratom and Mitra Distributing, companies marketing kratom, a potential source of opioids, with unproven medical claims.

FDA, innovator companies, and biosimilar developers maneuver over exclusivity, naming, interchangeability, and more.

Gore’s new flexible freeze containers are designed to protect high-value drug substances from container breakage or leakage.

As it investigates the root cause of an impurity discovered in valsartan, FDA extends its studies to APIs with similar synthesis processes.

Sharing know-how can help resolve common bio/pharma technical challenges.

This article focuses on applying new and traditional techniques to design a cleaning process, ensure the surfaces are clean, and develop rinse solution analysis to continuously monitor cleaning performance.

Detailed process descriptions and robust documentation aid in compliance as well as training, says Siegfried Schmitt, principal consultant at PAREXEL.
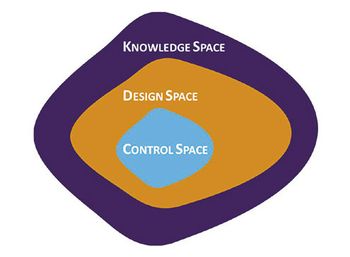
Using a QbD approach in the development and formulation of topical products will enable the drug developer to provide a robust control strategy for manufacturing.

The authors support the retiring of Ph. Eur. and USP heavy metal assays and propose a means of updating related specifications with minimal regulatory burden.

Increasingly stringent audit requirements will make third-party accredited GMP audits critical for pharmaceutical excipients and raw materials.

Sample preparation-specifically, API extraction and dilution-can introduce errors. Use of best practices and automation can reduce variability.

PDA Technical Report 80 (TR 80): Data Integrity in Laboratory Systems is the first in a series of three technical reports PDA will publish on data integrity.

A wave of potentially impure drugs and APIs from China, India, and other foreign firms has prompted recalls and FDA warning letters.

The company is voluntarily recalling one lot of Hydrochlorothiazide Tablets USP 12.5 Mg because of a labeling mix-up.

Pfizer Consumer Healthcare is recalling one lot of Children’s Advil Suspension Bubble Gum Flavored 4 FL OZ Bottle because of improperly marked dosage cups.

FDA proposed in a Federal Register notice that bumetanide, nicardipine hydrochloride, and vasopressin should not be included on the list of bulk drug substances that outsourcing facilities may use in compounding.

The Senate approved a $159-million budget increase for FDA, to bring its resources up to $5.4 billion for 2019, including more than $2 billion in user fees.

FDA sent a warning letter to Kyowa Hakko Bio Co., Ltd. after inspectors found data integrity problems at the company’s Yamaguchi, Japan facility.

King Bio is recalling several of its kids and infant products due to possible microbial contamination.

FDA approved the first generic version of EpiPen and EpiPen Jr (epinephrine) auto-injector.

FDA sent a warning letter to Apotex Research Private Limited after investigators found current good manufacturing practice violations.

FDA published a resource guide to promote responsible opioid prescribing in the treatment of animals.

Westminster Pharmaceuticals, LLC is voluntarily recalling Levothyroxine and Liothyronine after FDA issues import alert affecting the active ingredient.