
Understanding the differences in inspection processes is the key to successful global expansion, according to Siegfried Schmitt, PhD, vice-president Technical, PAREXEL Consulting.

Understanding the differences in inspection processes is the key to successful global expansion, according to Siegfried Schmitt, PhD, vice-president Technical, PAREXEL Consulting.

FDA plans to support initiatives to ensure that all medicines are safe, effective, and of high quality.
Shortages of life-saving drugs are a regulatory and industry concern. Proper process development may help to ensure drug supply.

While pharma is proving its capabilities to develop novel therapies, the industry still needs to work on manufacturing innovation.

As Brexit rapidly approaches, the lack of clarity around the regulatory balance between the regions could lead to a reduction in standards.

Intellectual challenge, work/life balance, compensation, and an unclear business outlook create uncertainty among European bio/pharma employees.

Core functions and those funded by FY 2018 user fees are continuing, and 59% of the Agency’s staffers are being retained.

FDA sent a warning letter to Roche’s Genetech for marketing unapproved stem cell products and puts other stem cell firms and providers on notice.

Despite ongoing efforts to address drug shortages, FDA now sees a rise in active shortages and in the duration of supply problems.

Agilent and other partners are funding development of Tapestri, a single-cell sequencing platform designed to help predict cancer relapse in individual patients and show the efficacy of gene-editing experiments.

The agency sent a warning letter to Cao Medical Equipment Co., Ltd. after inspectors found CGMP violations at the company’s Langfang, Hebei facility.

Asclemed USA Inc., dba Enovachem Pharmaceuticals is recalling the product due to labeling that incorrectly states that stoppers do not contain latex.

FDA has withdrawn the proposed rule that would have allowed generic-drug makers to independently update and distribute new safety information in drug labels.

New FDA guidance developed to identify lapses in data integrity and promote best practices.

FDA cites Zhejiang Huahai Pharmaceutical in valsartan impurity investigation.

FDA has approved Truxima (rituximab-abbs), a biosimilar to Roche’s anti-cancer biologic, Rituxan (rituximab).
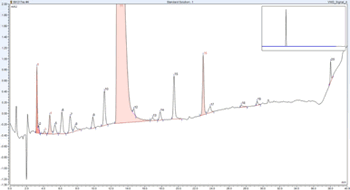
Integration of two separate chromatography data systems boosts workflow efficiency.

The proposal from the Centers for Medicare and Medicaid Services (CMS) proposes to give plans more flexibility to limit coverage of certain drugs.

A required time frame should not be the driving force behind root cause investigations, says Susan Schniepp, executive vice-president of Post-Approval Pharma and Distinguished Fellow, Regulatory Compliance Associates.
Microbial identity data can be critical for determining contamination sources.

Bio/pharma companies cannot spell success without solving all elements of quality programs.

Success depends on supplier communication and transparency, but it’s up to buyers to demand the right information and to look at the vendor’s overall business goals.

Simplified role-based training can lead to better quality metrics and compliance.

Once described as “throwing processes over the wall,” tech transfer is evolving into close collaboration and communication, as potential problems are considered sooner, and new technology is applied. Joseph Szczesiul, director of technical services for UPM Pharmaceuticals, shares best practices.

Drawing on practical experience, the authors examine key questions and answers about various aspects relating to the enhanced approach for analytical procedure lifecycle management.