
ICH elemental impurities guideline sets a global policy for limiting metal impurities in drug products and ingredients.

ICH elemental impurities guideline sets a global policy for limiting metal impurities in drug products and ingredients.

FDA drug approvals are up in 2014 with biologics drugs representing more than 25% of FDA approvals to date.

The agency publishes two guidance documents on providing regulatory submissions in electronic format.

The agency expresses its support for the adaptive pathway approach to bringing new drugs to patients.

FDA announced the approval of Gardasil 9 for the prevention of certain diseases caused by nine types of HPV, five more than the previously FDA-approved Gardasil.

US Department of Health and Human Services announced a declaration to provide immunity to legal claims made in the US in relation to three investigational Ebola vaccines.

The guidance seeks to help generic and biosimilar drug sponsors gain access to reference products from brand-name manufacturers.

Amgen's bispecific T-cell engager (BiTE) antibody constructs help the body fight malignant cancer cells.

Generic drug manufacturing is no longer the only driver for growth in India's pharmaceutical market as more companies start investing in R&D.

Enhanced R&D efforts and the growing manufacture of finished-dosage drugs in India will shape the country's future success, according to a new report from CPhI.

CytRx receives formal written communication from FDA on a prior decision for a partial clinical hold for CytRx's trials involving chemotherapeutic agent aldoxorubicin.

CDMO Vetter announced the addition of a new flexible serialization service, introduced in response to stricter packaging regulations calling for drugs to be serialized as a means to fight counterfeits.

The spate of drug scandals may alter the relationship between manufacturers and research institutions, and reshape Japan’s clinical research industry.

Ranbaxy and Epirus announce the launch of India's first biosimilar for Remicade.

Indian manufacturers are moving towards high-value, low-volume work, with complex chemistry and intellectual property challenges.

Lung Therapeutics announced that it received Orphan Drug Designation for LTI-01, an injectable designed for the treatment of loculated pleural effusion.

Dual sourcing is one of many possible solutions to securing the supply chain.

The authors explore and define common industry approaches and practices when applying GMPs in early development.

The author proposes a quality management system that uses the power of executive management to promote a positive quality culture.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses the requirements for good distribution practices.

Sponsor response to FDA's breakthrough program has exceeded FDA expectations, but puts pressure on manufacturers to address formulation, stability and quality production issues very early in development.
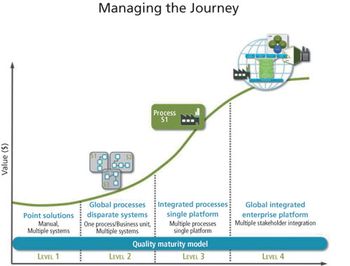
Enterprise quality management systems can help shift the quality emphasis from corrective to preventive actions.

Manufacturers face regulatory overhaul, while brand-generic debates escalate over biosimilars and labeling changes.

Aurobindo Pharma USA issued a voluntary nationwide recall of Northstar Label Gabapentin Capsules, USP 300 mg, due to complaints of empty capsules.

The Baxter recall in the US of one lot of highly concentrated potassium chloride is due to a mislabeled overpouch.