
Pharmaceutical Technology spoke with Tim Kearns, pharmaceutical and medical devices manager at Videojet.

Pharmaceutical Technology spoke with Tim Kearns, pharmaceutical and medical devices manager at Videojet.

The House Energy and Commerce Committee gave unanimous approval to the landmark 21st Century Cures Act reform bill on May 21, 2015.

Optical inspection equipment for packaging was displayed at INTERPHEX 2015.

The new guideline replaces the previous version-CHMP/EWP/240/95 Revision 1.

The former New England Compounding Center will pay $200 million to victims and creditors for the 2012 outbreak of meningitis that killed 64.

Raplixa fibrin sealant, the first approved spray-dried biologic, is a hemostatic agent that helps control bleeding from small blood vessels during surgery.

FDA concludes Orkambi demonstrates a clinical benefit over placebo, but questions the magnitude of the improvement.

Pluristem Therapeutics announces that Japan’s Pharmaceuticals and Medical Devices Agency agreed with its methods for the manufacture of PLX-PAD cells.

The new Kaye Validator AVS combines accurate sensor measurements with all GMP requirements for calibration and traceability.

The agency publishes draft guidance answering industry questions about the Biologics Price Competition and Innovation Act.

FDA issues a Form 483 following inspection of Impax Laboratories' Hayward, CA facility.

The basic principle behind QbD is that quality should be built into a product with an understanding of the product and process by which it is developed and manufactured along with a knowledge of the risks involved in manufacturing the product and how best to mitigate those risks.

Novartis announces that its lung cancer drug, Zykadia, gained European Union approval.

Johnson & Johnson announces partnership with NYU School of Medicine to launch a compassionate care committee for individual patient requests of investigational drugs.

The US Court of Appeals granted Amgen’s request to block Novartis’ Neupogen biosimilar, Zarxio, from the US market until the court resolves litigation between the two companies.

The company voluntarily recalls select lots of Adrucil due to particulate matter.

FDA cites Yunnan Hande Bio-Tech for cGMP violations related to data collection and security.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how to keep up with changing regulations.

The European Pharmacopoeia defines the format and content of monographs for biologicals to keep pace with recent approaches and meet the needs of its users.

Compliance with the new traceability requirements necessitates an understanding of how and when to begin implementing changes in an ever-evolving industry.
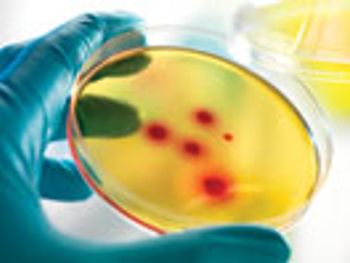
The author reports results of evaluations and concludes that a disinfectant composed of a low-concentration suspension of silver ions is completely sporicidal with only a one-minute contact time.

Pharma can boast of big-picture successes, but needs to work on operational issues.

The restructuring of the International Conference on Harmonization, which is expected to begin in late 2015, could have a significant impact on the way pharmaceutical regulations are harmonized worldwide.

A complex and evolving set of regulations challenges drug manufacturers to understand and implement serialization requirements.

The revised "21st Century Cures” proposal includes provisions for research for continuous manufacturing, biomarker development, and NIH funding.