
Through the acquisition, Avantor will have access to RIM’s Changzhou, China, facility, making it Avantor’s first single-use production plant in the AMEA region.

Through the acquisition, Avantor will have access to RIM’s Changzhou, China, facility, making it Avantor’s first single-use production plant in the AMEA region.

The companies are expanding their collaboration to extend the drug substance manufacturing of Moderna’s COVID-19 vaccine with the addition of a new manufacturing line at Lonza’s Geleen, Netherlands, site.

Moderna has initiated a rolling submission process with FDA for a biologics license application for its mRNA COVID-19 vaccine for individuals 18 years of age and older.

Expanded interest in advanced drug manufacturing and continuous production methods calls for more flexible production systems and regulatory policies.
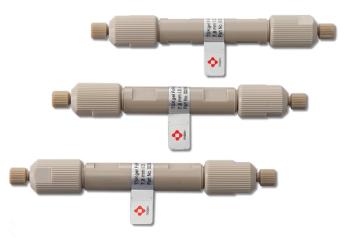
Tosoh Bioscience introduced the TSKgel FcR-IIIA-5PW HPLC column, a semi-preparative affinity column based on a recombinant FcγRIIIA receptor ligand bonded to porous 10 µm polymethacrylate particles.

PPD has opened its new multipurpose clinical research laboratory in Suzhou, China, which offers bioanalytical, biomarker, and vaccine sciences services for China and the global market.

Expected to be completed by 2022, the new addition is part of the Samsung Biologics’ long-term strategy to become a fully integrated global biopharmaceutical company.

In the face of a growing clamor for greater worldwide access to COVID-19 vaccines, biopharma companies are promising to expand distribution of free and low-cost preventives to curb the pandemic globally.

The Discovery Labs has signed a foundational lease with the University of Pennsylvania Gene Therapy Program to build a new life sciences cluster in King of Prussia, Pa.

Moderna and Aldevron have expanded their existing collaboration to support Moderna’s COVID-19 vaccine program as well as additional clinical programs in Moderna’s pipeline.

Biogen and Ginkgo Bioworks have partnered to develop a next-generation AAV production platform to accelerate Biogen’s gene therapy drug development efforts.

Codagenix and Univercells have partnered on a research collaboration to pursue an undisclosed, high-priority human vaccine target.

In the study, which enrolled 3700 US participants ages 12 to less than 18 years, no cases of COVID-19 were found in participants who had received two doses of the Moderna COVID-19 vaccine.

The new line can fill prefilled syringes, cartridges, and vials using ready-to-use components.

Evonik has introduced the EUDRACAP, a portfolio of easy-to-handle capsules to fast-track speed to market for oral drug products in the early development stage.

Almac Sciences is investing in the expansion of its continuous flow chemistry equipment as an additional project to its previously announced £5-million (US$7-million) laboratory expansion.

Samsung Biologics plans to support the production of hundreds of millions of doses of Moderna’s vaccine to supply to markets outside of the United States beginning in the third quarter of 2021.

Under the terms of the agreement, Thermo Fisher will construct and operate a 44,000-ft2 cell therapy development, manufacturing, and collaboration center on UCSF's Mission Bay campus.

Univercells will introduce the intermediate capacity scale-X carbo system to NIBRT’s facility for hands-on demonstration and training in CGT.

Sandoz plans to strengthen its European antibiotics manufacturing network by expanding its production capabilities in Kundl, Austria, and Palafolls, Spain.

BD’s new high-tech manufacturing facility in Zaragoza, Spain, will create up to 600 jobs by 2030.

Colorcon’s new suite will provide clients with capabilities to develop prototype tablets or capsule dosage forms when API quantity is limited or the API potency requires a higher level of protection.

Sanofi and GlaxoSmithKline reported that their adjuvanted recombinant COVID-19 vaccine candidate demonstrated strong immune responses across all adult age groups in a Phase II trial.

Charles River plans to enhance its gene therapy capabilities through the acquisition of Vigene Biosciences.

WuXi Biologics and WuXi STA have established a joint venture company named WuXi XDC to provide contract development and manufacturing of bioconjugates, including antibody-drug conjugates.