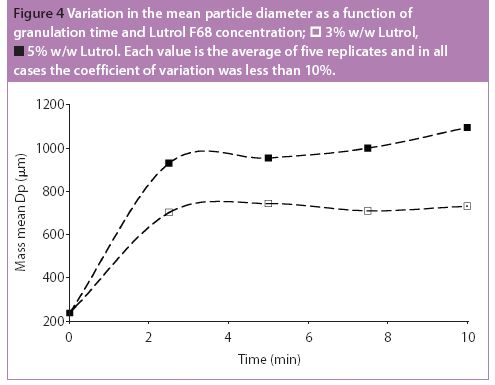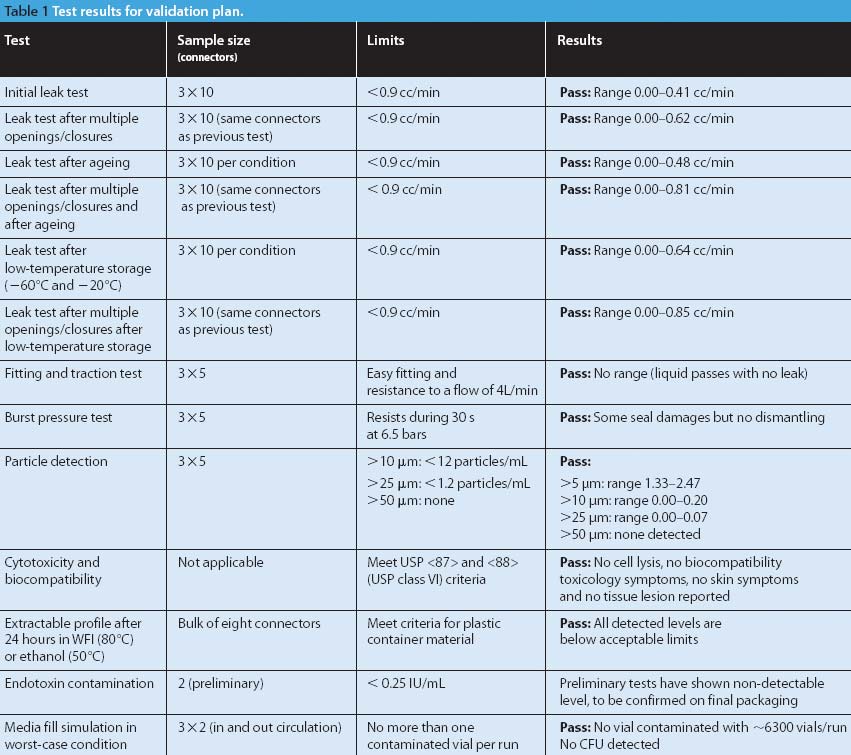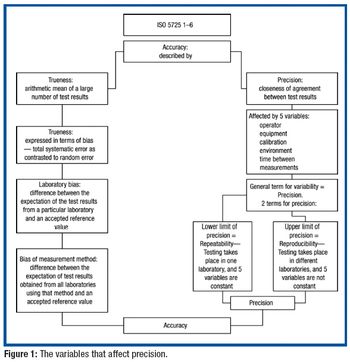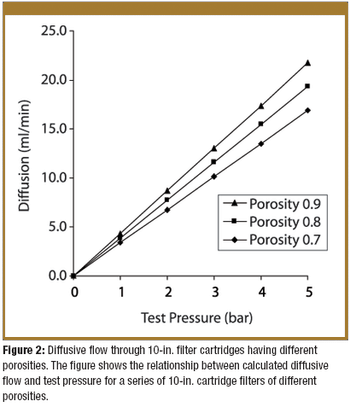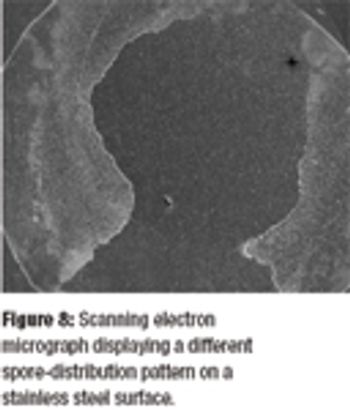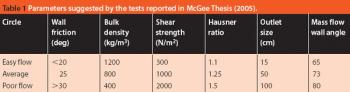
Predicting the flow characteristics of powders during manufacture is especially important for the pharmaceutical engineer. Getting the powder flow wrong can be highly disruptive to plant performance and productivity, particularly where equipment has to be taken off-line and stripped down for cleaning out blockages. The flow behaviour of the individual ingredients may be well known, but as these are blended and reacted their flow properties can change.