
Freeze dryer internal surfaces become contaminated, post cycle, by the ablation of API and excipient from the freeze?drying cake.

Freeze dryer internal surfaces become contaminated, post cycle, by the ablation of API and excipient from the freeze?drying cake.

Lyophilization is an expensive process that demands high investments in technology, but for many products, pharmaceutical freeze drying is currently the only method to ensure durability.

Technological advances and regulatory documents, such as Annex 1 of the EU Guideline for Good Manufacturing Practice, have encouraged the pharmaceutical industry to install various new technologies into their production lines.

When freeze drying biological materials, the major concern is achieving product consistency both within a batch and between batches.

Despite numerous benefits, computer modelling has mainly remained in the domain of academic research.

The most important consideration when choosing a freeze dryer is to ensure the system is fit for both today's applications and future needs.

The World Health Organization released new guidelines this week for the treatment of malaria and the first-ever guidelines on procuring safe and efficacious antimalarial drugs.

Exelixis And XenoPort Announce Job Cuts; GSK Dedicates India Facility; And More.

The US Food and Drug Administration recently published guidance for the characterization and qualification of cell substrates, viral seeds, and other biological materials used to manufacture viral vaccines for human use.

A UK consortium completed pilot runs of a pharmaceutical-safety program that uses electronic tracking and authentication to ensure the safety of drugs in the supply chain.

The US Food and Drug Administration and the European Medicines Agency are implementing a streamlined process to help regulators better identify and share information regarding orphan-drug and biologic products.

Merck KGaA has agreed to acquire Millipore for EUR 5.3 billion ($7.2 billion).

Last week, the US Food and Drug Administration and the National Institutes of Health unveiled plans to establish a Joint Leadership Council to address important public-health issues.

Astellas Pharma Makes Bid for OSI Pharmaceuticals; Ringo Retiring From Pfizer; And More.

Top pharmaceutical and biotech companies should expect a major slowdown in sales growth between 2008 and 2014, according to business analysts Datamonitor.
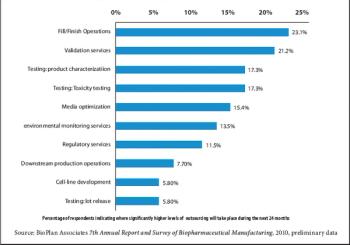
Strategic rather than tactical considerations are driving biopharmaceutical outsourcing.

Following its $68-billion acquisition of Wyeth, Pfizer is integrating its manufacturing and outsourcing activities (Podcast).
Editors' Picks of Pharmaceutical Science & Technology Innovations

CMOs' business model has all of the flaws of the captive model it is meant to replace.

Could President Obama's tax reform, which is targeted at reducing outsourcing, endanger India's contract-services industry?

Emerging markets remain an important element in the strategies of pharmaceutical companies and their suppliers.

Pre-Interphex 2010 Product Releases.
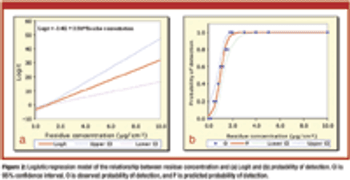
Current methods for establishing visible residue limits (VRLs) are not statistically justifiable. The author proposes a method for estimating VRLs based on logistic regression.
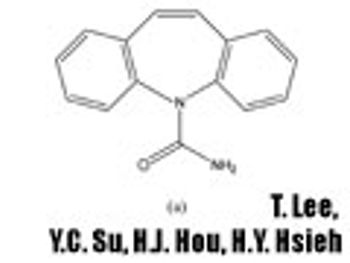
In Part I of this article, the authors describe the materials and methods used in developing a screening strategy to accelerate the preparation and characterization of spherical agglomerates by spherical crystallization.

To best carry out the vision of Hatch-Waxman, Congress must act now on biogenerics.