
The US Court of Appeals granted Amgen’s request to block Novartis’ Neupogen biosimilar, Zarxio, from the US market until the court resolves litigation between the two companies.

The US Court of Appeals granted Amgen’s request to block Novartis’ Neupogen biosimilar, Zarxio, from the US market until the court resolves litigation between the two companies.

The company voluntarily recalls select lots of Adrucil due to particulate matter.
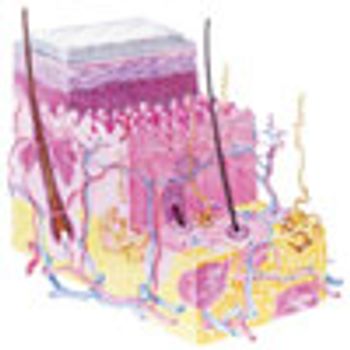
Advances in transdermal drug delivery, particularly with microneedles, are enabling a wider range of drugs to be delivered through the skin.

Novo Nordisk announces that it will invest DKK1.5 billion (US$224 million) in a haemophilia treatment manufacturing facility in Denmark.

Capsugel extends inhaled biotherapeutics delivery capability to Phase 2 clinical trials.

FDA cites Yunnan Hande Bio-Tech for cGMP violations related to data collection and security.

Intellectual property lawyers estimate biosimilar litigation will swell as early as 2018.
Adjusting the tablet press and its systems can prevent manufacturing and product quality problems.

The European Pharmacopoeia defines the format and content of monographs for biologicals to keep pace with recent approaches and meet the needs of its users.

Compliance with the new traceability requirements necessitates an understanding of how and when to begin implementing changes in an ever-evolving industry.

Thermo Scientific’s TSX ultra-low temperature freezer is designed to lower its impact on the environment with the use of natural refrigerants, using up to 50% less energy than conventional refrigerant ultra-low freezers.

Transitioning from paper records to electronic batch records decreases costs and increases efficiency.
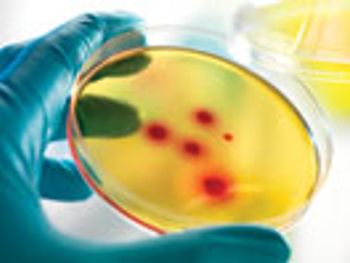
The author reports results of evaluations and concludes that a disinfectant composed of a low-concentration suspension of silver ions is completely sporicidal with only a one-minute contact time.
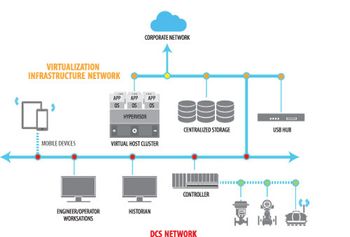
Virtualization has been mainstream in information technology (IT) for decades.


A complex and evolving set of regulations challenges drug manufacturers to understand and implement serialization requirements.

Pharmaceutical companies should take into consideration intellectual property protection when outsourcing the packaging of their products.

FDA releases long-awaited guidance documents regarding the assessment of biosimilarity.

CDMO Vetter produces identifiable labeling for a top-ten pharmaceutical company.

IDT Biologika receives 2015 Facility of the Year Award or facility integration from ISPE.

With the Indian pharmaceutical industry on the rise, manufacturing businesses are working together with European and American partners to harness their longstanding experience and reputation in cleanroom manufacturing for a broader pharmaceutical manufacturing marketplace.

Catalent’s Zydis technology will be used to develop thermo-stable and cold-chain independent vaccines.
FlexiBulk tip packs save space and effort in the laboratory

Originator product manufacturers will have to update and improve their processing platforms to stay competitive with the biosimilars coming to market.

Prequalified manufacturing suites could benefit from a new business model, say some industry executives.