
BioSC Lab is the first in a line of next-generation chromatography equipment for protein purification in batch and continuous modes.

BioSC Lab is the first in a line of next-generation chromatography equipment for protein purification in batch and continuous modes.

The agency clarifies its requirements for allowable excess volume and labeled vial fill size in injectables and biologics.

Reach Separations has announced plans to double its laboratory space at its Nottingham facility as a result of increased demand for its specialist purification services.

Monoclonal antibodies can facilitate the entry of radiopharmaceuticals into cells, David Scheinberg said at BIO 2015.

The agency takes action against websites that illegally sell unapproved medications.

NIH provides an interim corrective action plan to correct deficiencies found in its Clinical Center Pharmaceutical Development Section.

EXCiPACT announces that DQS awarded Grace GmbH an EXCiPACT Certificate for its Worms, Germany excipient-manufacturing site.

Lonza’s planned facility will be used to develop and manufacture viral gene therapies and virally modified cell therapies

Presenters at IVT's Microbiology Week discussed best practices and recent guidance publications for microbial control in sterile and non-sterile pharmaceutical processes.

Although full traceability is not required by law in the US until 2023, companies could benefit from implementing it now.

Experts at Eppendorf discuss common challenges in cell culture and share insights on possible solutions.

Nic Michel, General Manager of Pharma Technology, Inc., spoke with Pharmaceutical Technology about the application of external lubrication in tablet production.

Catalent’s Singapore facility is awarded GMP certification.

Vetter completes on-site expansion activities of visual inspection and in-process control at Chicago facility.

West Pharmaceutical began construction on its component manufacturing facility located in Waterford, Ireland.

A new GMP facility in Massachusetts will produce enzymes and other reagents for in-vitro diagnostics.

With drug development trends shifting towards personalized medicines, new technologies are needed for the manufacture of these highly sensitive drug products.

The exclusive manufacturing collaboration will establish production for adeno-associated virus gene-therapy treatments incorporating REGENXBIO’s NAV Technology.

Lyophilization Services of New England announces inspection approvals that will allow its Bedford, NH facility to begin manufacturing commercial drugs for US distribution.
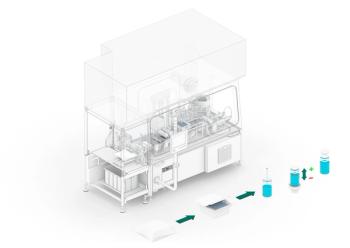
Groninger has developed FlexPro 50, an isolator system for aseptic processing of nested syringes, cartridges, and vials. FlexPro 50 is capable of achieving outputs of up to 5000 containers per hour.

The increasing number of biopharmaceuticals in the development pipeline has created the need for packaging technologies with enhanced safety features, especially for sensitive biologic drugs.

Designed with safety standards in mind, flowtherm Ex incorporates a number of connectable sensors, such as vane wheel, vortex, thermal, Pt100, and other physical sensor with analog output, thereby allowing a range of applications.

The design of the shovel means that less drive power is required compared with a standard shovel, thus resulting in substantial energy savings.

Talks will explore key trends and issues in the pharmaceutical space and address the “latest industry buzz.”

Optima Pharma is exhibiting a range of technologies from sterile-filling and freeze-drying solutions to isolators and restricted access barrier systems.