
This article examines multi-tip tool technology for pharmaceutical tablet compression and the process control and validation issues that must be carefully evaluated to assess the potential for success.

This article examines multi-tip tool technology for pharmaceutical tablet compression and the process control and validation issues that must be carefully evaluated to assess the potential for success.

Tools help improve understanding of excipient risk in formulating OSD drugs.

This paper discusses what causes cross-linking, how cross-linking is addressed with addition of enzymes, and consideration for occasional high results that can be obtained during release testing.

Advanced dynamic process control of a fluid-bed granulation process using PAT data improves product quality.

A coalition establishes a secure, direct link between a physical tablet and a digital backend for supply chain integrity.

Improving operator skills using flexible, online training benefits pharmaceutical tableting operations.

CPI, the University of Strathclyde, GSK, and AstraZeneca Collaborate on a continuous direct compression digital twin for pharmaceutical formulation optimization.

Establishing OEL data and ensuring appropriate engineering controls are crucial aspects of safe handling.

At-line NIR measurements can replace a laboratory HPLC measurement for API content in oral solid-dosage drug manufacturing.

In this interview, the main uses and benefits of NIR spectroscopy in tableting are discussed.

Early adopters of continuous manufacturing approaches shared their plans and some of their experiences at the 11th annual Charles Jarowski Symposium in Industrial Pharmacy.

Homogeneity of the powder blend is crucial for solid-dosage drug manufacturing.

Lonza plans to implement MES software to accelerate paperless, 24/7 production of drug capsules.

The expansion of Lonza’s Tampa site includes development and manufacturing capabilities for oral solid-dose drugs.

A presentation at the Leistritz Pharmaceutical-Nutraceutical Extrusion Seminar explained how simulation tools benefit the development process.

StarTab from Colorcon was designed to ensure functionality and stability for direct compression tableting.

The UK’s Centre for Process Innovation is collaborating with partners GSK and AstraZeneca to establish a continuous wet granulation manufacturing facility for small-scale development of oral solid-dosage pharmaceuticals.

Educational sessions on the INTERPHEX show floor will include a panel discussion on implementing continuous manufacturing.

3D printing offers a new design freedom for bio/pharmaceutical manufacturing: whether for “printing” a solid-dosage drug or for creating a piece of equipment for bio/pharmaceutical laboratories or manufacturing facilities.

In OSD continuous manufacturing, flexible batch sizes can optimize supply, but equipment and processing challenges are still being addressed.

When specifying an automated powder transfer system for vacuum conveying of pharmaceutical powders, consider material properties, facility constraints, and designs to mitigate explosion.
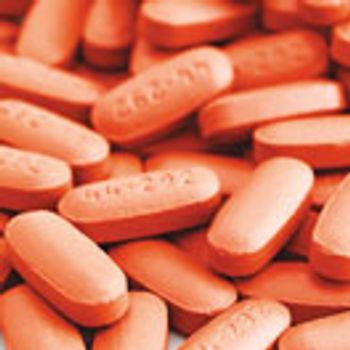
Sticking and picking are common problems in tablet manufacturing. Considering tablet compression issues before tablet designs are finalized can prevent unanticipated problems during scale-up and full-scale production.

Flexibility and well considered manufacturing approaches could help tablet manufacturers face the increasing pressure resulting from the shifting bio/pharma development landscape.

Market demand and regulatory guidance continues to promote improved medication design.

Serialization-ready packing equipment and services to manage data assist companies with DSCSA compliance.