
Roche's plans for a major biologics manufacturing expansion is the latest investment by Big Pharma

Roche's plans for a major biologics manufacturing expansion is the latest investment by Big Pharma

Roche plans multimillion biologic manufacturing expansions in California, Switzerland, and Germany.

Scientific Digital Imaging's Synbiosis Division introduces SynStats statistical analysis software.

Advanced Biosciences Laboratories will support the development and production of a novel Shigella vaccine candidate for PATH.

USP seeks input from stakeholders on new and revised standards to mitigate extractables and leachables in plastic packaging systems.

Industry experts discuss the importance of characterization studies during biosimilars development and related analytical methods.

The Biomanufacturing Research Program (BioMAN) at Massachusetts Institute of Technology (MIT) has received $10.4 Million from the Defense Advanced Research Projects Agency (DARPA) to develop new technologies and manufacturing platforms that will provide an emergency supply of medicines for front-line military medics.

Merck & Co gets FDA approval to manufacture bulk varicella at its in Durham, North Carolina for use in chickenpox and shingles vaccines.

Prequalification of Sanofi Pasteur?s Menomune vaccine makes it eligible for purchase by United Nations agencies.

Avacta?s Optim 2 instrument provides a practical solution for determining the conformational stability of adjuvant bound vaccines, eliminating the need for awkward and time consuming standard analytical techniques. [more?..]

Industry experts discuss the requirements and challenges involved in getting a biosimilar product from bench to launch.
Extensive comparability testing is required to ensure that biosimilars have comparable profiles to their reference products.
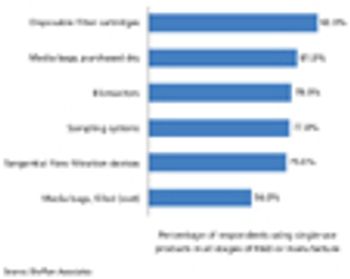
Advances in techniques and single-use systems are revolutionizing vaccine manufacturing.

A new method of vaccine design, called the Multiple Antigen Presentation System (MAPS), may result in vaccines that bring together the benefits of whole-cell and acellular or defined subunit vaccination.

Novartis and Biological E, a biopharmaceutical company based in India, have entered into an agreement that aims to deliver affordable and accessible vaccines for typhoid and paratyphoid A fevers to developing countries and thereby address the unmet medical need in endemic regions.

Conjugated vaccines are meeting the need for longer-lasting immune responses, but the production process is complex, and manufacturers are looking for simpler solutions.

Modular containment room at Belfast facility allows studies of biologics and vaccines.

GSK expands its vaccines platform technology expertise by acquiring Okairos, a Swiss-based company.
The authors describe a holistic and integrated approach to focus on the linkage of the prefilled syringe with the four phases of product design, development, operation, and control.

Catalent Pharma Solutions, a provider of drug and biologic development services, delivery technologies and supply solutions, has officially opened a new biomanufacturing center of excellence in Madison. The facility, which was constructed in response to customer demand, is expected to quadruple Catalent's current biologics manufacturing capacity and extensively utilise single-use technology for greater flexibility and efficiency. It will allow the company to extend its offerings in the biologics sector while enhancing the efficiency and output of its proprietary GPEx cell line engineering technology as well as other mammalian cell lines.

BioOutsource, a contract testing services provider to the biopharmaceutical industry, has opened a new facility in Cambridge, Massachusetts. The facility marks the company's first North American offices, which were opened in response to increasing demand for the company's bioanalytical and biosafety services, particularly in the field of biologic and biosimilar characterisation.

A Q&A with Tony Hitchcock, head of manufacturing at Cobra Biologics.

America's biopharmaceutical companies are using biological processes to develop 907 medicines and vaccines targeting more than 100 diseases, according to a new report.

Vaccine development is benefiting from manufacturing advances and support for global health.

Protein Sciences announced that the Biomedical Advanced Research and Development Authority (BARDA) will continue to support the company?s influenza vaccines program.