
Single-use technologies, modular systems, and robots are on the rise.

Single-use technologies, modular systems, and robots are on the rise.
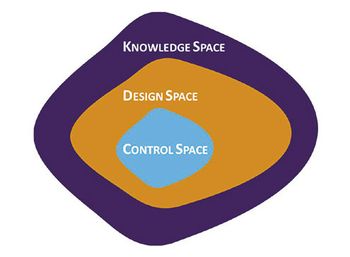
Using a QbD approach in the development and formulation of topical products will enable the drug developer to provide a robust control strategy for manufacturing.
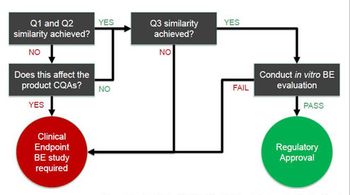
This article examines IVBE testing requirements for topical creams and explores some of the analytical techniques necessary.

The human skin protects the body from physical, mechanical, and chemical insults while preventing endogenous water loss. This function is predominantly achieved by a thin (10–30 µm) cornified outermost layer-the stratum corneum (SC)-generated through terminal differentiation of the basal epidermal keratinocytes. The stratum corneum protects the human body, but also severely limits drug delivery into and across the skin.

Thixotropic gels, thermosoftened systems, and self-emulsifying systems have expanded the range of potential excipients for liquid-filled hard capsules (LFHC).

API-in-capsule approaches enable pharmaceutical companies to quickly assess new drug candidates with reduced API consumption and to increase speed to clinic.
Immobilizing the antibodies on a solid-phase support, such as a resin, and carrying out the conjugation of the payload-linker while the antibodies are bound to that support will prevent aggregation at its source.

Protagen Protein Services, a CRO, now offers quicker and more accurate characterization of biomolecular stability using differential scanning calorimetry (DSC).

The European Commission (EC) has approved Novartis’ chimeric antigen receptor T cell (CAR-T) cell therapy Kymriah for the treatment of B-cell acute lymphoblastic leukemia and relapsed or refractory diffuse large B-cell lymphoma after two or more lines of systemic therapy.

Preliminary survey results rank the United States, Germany, and Japan as tier-one nations for bioprocessing performance and potential.

The Coalition for Epidemic Preparedness Innovations (CEPI) and vaccine manufacturer IDT Biologika will collaborate to develop and manufacture a vaccine against Middle East Respiratory Syndrome Coronavirus (MERS-CoV).

Experts from the Partnership for Research on Ebola VACcination report on progress and uncertainties regarding a safe and effective Ebola vaccine.

The National Institute of Allergy and Infectious Diseases began a first-in-human trial of an experimental live, attenuated Zika virus vaccine.

The acquisition of Juniper expands and strengthens Catalent’s offerings in formulation development, bioavailability solutions, and clinical-scale oral dose manufacturing.

FDA approved the first generic version of EpiPen and EpiPen Jr (epinephrine) auto-injector.

The acquisition is expected to support Emergent BioSolutions’ focus on public health threats and emerging infectious disease.

The company’s purpose-built facility in Norwich, United Kingdom specializes in plant-based expression of proteins, metabolites, and complex natural products.

The companies entered a multi-year R&D collaboration to develop mRNA-based flu vaccines.

Drug product approval from FDA follows previous approvals from European and Japanese authorities.

The new drug, Onpattro (patisiran), by Alnylam Pharmaceuticals, is in a new class of drugs called small interfering ribonucleic acid (siRNA) treatment.

Ebola vaccinations by the World Health Organization began in North Kivu, Democratic Republic of the Congo, one week after the country’s latest outbreak.

Pharmaceutical companies are developing new strategies to address the ever-increasing development costs for new drug therapies and maximize the value of their existing drug portfolio.

The European Commission (EC) approved Pfizer’s Trazimera (trastuzumab), a biosimilar to Roche’s Herceptin, to treat certain breast and gastric cancers.

The companies will collaborate on the discovery, development, and commercialization of cell therapies for cancer.

Formulation characteristics must be optimized, following device selection, to achieve the desired performance for the capsule loaded in the dry powder inhaler.